【综述】| 抗体药物偶联物在HER2阳性晚期胃癌中的应用进展和展望
时间:2023-09-25 17:15:45 热度:37.1℃ 作者:网络
[摘要] 胃癌是一种异质性和侵袭性极高的恶性肿瘤,在全球范围内其发病率居第5位,死亡率居第3位。多数患者确诊时已处于进展期,预后极差。全身治疗是目前晚期胃癌最主要的治疗方式,人表皮生长因子受体2(human epidermal growth factor receptor 2,HER2)是HER2阳性胃癌的重要治疗靶点。随着HER2靶向药物和治疗方式的不断优化,一些胃癌患者已从中获益。但是耐药发生率高、不良反应严重仍是限制HER2靶向药物应用的瓶颈。因此,开发新型抗肿瘤药物对改善HER2阳性胃癌患者的长期生存具有重要意义。抗体药物偶联物(antibody drug conjugate,ADC)是一类新型、高效的抗肿瘤药物,由特异性靶向单克隆抗体、化学接头和小分子细胞毒性有效载荷组成,强大的治疗效果和适度的组织毒性是其主要优势。近年来,ADC在HER2阳性晚期胃癌的靶向治疗领域进展颇丰。首先,经过多年发展,包括DS-8201、RC48等在内的多种ADC已用于胃癌的二线及后线治疗。其次,随着ADC生物工程技术的进步,包括高药物抗体比例、可切割型连接子、能引发旁观者效应的毒性载荷等,使新型ADC能够在针对特定靶点肿瘤的治疗中发挥更为显著的治疗效果,其中一些ADC还具备多靶向性,能针对多个特异性靶点发挥抗癌功效。同时,ADC的研发已到达第三阶段,新一代ADC通过位点特异性偶联技术,其均质单一性更高,细胞毒性载荷更高效,精准度更高,非靶向毒性作用更低。此外,ADC和免疫检查点抑制剂(immune checkpoint inhibitor,ICI)组成的“靶免联合”治疗可能是晚期胃癌一种有前景的治疗策略。本文就靶向治疗时代ADC在HER2阳性晚期胃癌患者中的应用和最新研究进展进行综述,并讨论ADC联合ICI在HER2阳性晚期胃癌中的治疗前景和治疗过程中面临的挑战。
[关键词] 人表皮生长因子受体2;晚期胃癌;抗体药物偶联物;分子靶向治疗;进展
[Abstract] Gastric cancer is a malignant tumor with high heterogeneity and invasiveness. Its incidence rate ranks fifth in the world, and its mortality ranks third in the world. Most patients are in a state that cancer cannot be removed by surgery when symptoms appear. At present, systemic treatment is the main treatment for advanced gastric cancer, and human epidermal growth factor receptor 2 (HER2) is one of the important treatment targets for HER2 positive gastric cancer patients. With the continuous optimization of chemotherapy regimen and targeted drugs, the prognosis of some HER2 positive gastric cancer patients has improved significantly. However, the high incidence of drug resistance and high toxicity and side effects are still the bottlenecks limiting the application of HER2 targeted drugs. Therefore, the development of new anti-tumor drugs is of great significance to improve the long-term survival of HER2 positive gastric cancer patients. Antibody drug conjugate (ADC) is a new and efficient anti-tumor drug, which is composed of specific targeted monoclonal antibody, chemical connector and small molecular cytotoxic payload. Its main advantages are strong therapeutic effect and moderate tissue toxicity. In recent years, ADC has set off a huge upsurge in the targeted treatment of HER2 positive advanced gastric cancer. First, after years of development, a variety of ADC including DS-8201 and RC48 have been used in the second- and second-line treatment of gastric cancer. Secondly, with the progress of ADC bioengineering technology, including high proportion of drug antibodies, cleavable linkers, toxic loads that can trigger bystander effect, the new type of ADC can play a more significant therapeutic role in the treatment of specific target tumors, and some of them also have multiple targets and can have anti-tumor effect on multiple specific targets. At the same time, the research and development process of ADC has reached the third stage. The new generation of ADC, through site-specific coupling technology, has higher homogeneity and uniformity, more effective cytotoxic molecules, higher accuracy and lower non-targeted toxicity. In addition, the "targeted immunotherapy" composed of ADC and immune checkpoint inhibitor (ICI) may be a promising treatment strategy for advanced gastric cancer. This article briefly reviewed the application and the latest research progress of ADC in HER2 positive advanced gastric cancer patients in the era of targeted therapy, and discussed the treatment prospects and challenges of ADC combined with ICI in HER2 positive advanced gastric cancer.
[Key words] Human epidermal growth factor receptor 2; Advanced gastric cancer; Antibody drug conjugate; Molecular targeted therapy; Progress
胃癌是颇具侵袭性和异质性的恶性肿瘤之一。约20%的胃癌患者存在人表皮生长因子受体2(human epidermal growth factor receptor 2,HER2)蛋白过表达或基因扩增。尽管HER2靶向药物已广泛用于晚期HER2阳性胃癌的一线、二线以及后线治疗,但患者预后不良、耐药率高仍是限制其使用的主要瓶颈。因此,开发新型靶向HER2抗胃癌药物尤为重要。
早在20世纪初,Paul Ehrlich等就首次提出了 “魔法子弹”的概念,并假设某些化合物可以直接通过肿瘤细胞内的一些特定靶点来发挥杀伤作用。理论上讲,这些化合物能够有效地杀死肿瘤细胞,而对正常细胞无伤害。其中一种方法是通过检测某些特异性过表达抗原,以区分肿瘤细胞和正常细胞,例如HER2、CD20、c-MET等,这些抗原的异常表达为单克隆抗体(monoclonal antibody,mAb)的特异性识别提供了可能。此后,随着杂交瘤技术的兴起,该领域得到迅速发展[1]。截至2022年底,全球已有15种抗体药物偶联物(antibody-drug conjugate,ADC)获批用于实体瘤和血液系统恶性肿瘤的治疗。同时,超过百种ADC候选药物处于临床研究的不同阶段。此外,随着治疗靶点和适应证的不断扩大,ADC正在引领靶向治疗的新时代,并有望在未来替代传统化疗,同时也为HER2阳性胃癌患者带来了新的希望。
ADC是由特异性mAb和高度杀伤力细胞毒性药物,通过特定连接点将靶标偶联起来的分子靶向生物制剂。具有小分子药物的细胞毒性与mAb的特异性识别两大优势,与常规化疗药物相比,具有精准的肿瘤杀伤特性,也能够选择性地降低药物脱靶引起的不良反应,进而更好地发挥抗癌作用。因此,ADC是未来抗肿瘤免疫治疗领域的一大热点,尤其是新靶点药物的设计、下一代ADC的开发以及ADC与免疫检查点抑制剂(immune checkpoint inhibitor,ICI)联合应用等方面,具有广阔的前景。
1 ADC在HER2阳性晚期胃癌中的应用
目前,国内外多个靶向HER2 ADC获批用于局部晚期胃癌患者的二线及后线治疗,在大量临床研究中显示出较好的治疗效果。
1.1 恩美曲妥珠单抗
T-DM1由trastuzumab、微管蛋白聚合抑制剂美坦辛衍生物通过硫醚连接子组成,药物抗体占比(drug antibody ratio,DAR)为3.5。其通过细胞膜受体介导的内吞作用将美坦辛衍生物递送至HER2过表达细胞内,通过与微管蛋白结合,阻止微管聚合和形成,使细胞周期停滞在有丝分裂中期,进而杀伤肿瘤细胞[8]。Mamounas等[2]在KATHERINE研究的亚组分析中发现,与trastuzumab相比,T-DM1能够使HER2阳性乳腺癌患者获益,并降低中枢神经系统的复发风险。Zhang等[3]发现T-DM1通过诱导HER2阳性肿瘤细胞凋亡以及保护性自噬,抑制其增殖。因此,T-DM1成为首个治疗HER2阳性乳腺癌的 ADC[4-5]。鉴于其对乳腺癌具有良好的治疗效果,在晚期胃癌中的疗效也备受关注。
一项对415例HER2阳性晚期胃癌或胃食管交界癌患者的随机、开放标签、Ⅱ/Ⅲ期研究[6]表明,T-DM1和紫杉醇治疗组患者的中位总生存期(overall survival,OS)分别为7.9和8.6个月(P=0.86),中位无进展生存期(progression-free survival,PFS)分别为2.7和2.9个月(P=0.31)。3~4级不良事件(adverse event,AE)发生率分别为59.8%和70.3%。其中,最常见的3~4级AE为贫血和血小板减少。此外,8例患者在T-DM1治疗后死亡。因此,T-DM1在HER2阳性晚期胃癌患者中的疗效具有较明显的局限性,可能与DM1膜渗透性较差,药物“旁观者”效应较低;药物过早在体内解离暴露,增加了T-DM1的非靶向毒性等因素有关。同时,胃癌原发灶和转移灶中HER2表达水平不同也与T-DM1疗效不理想密切相关。
1.2 曲妥珠单抗德卢替康
T-DXd(DS-8201)由trastuzumab通过可裂解四肽连接子与拓扑异构酶Ⅰ抑制剂DXd(喜树碱类衍生物)连接组成,其抑制效力与伊立替康活性代谢物SN-38相比高10倍。与T-DM1不同,T-DXd采用半胱氨酸偶联,DAR高达8。因此,能更有效地将细胞毒药物输送到肿瘤细胞内。此外,其有效载荷半衰期较短,显著增加了其抗体依赖的细胞介导的细胞毒作用(antibody-dependent cell-mediated cytotoxicity,ADCC)效应,避免了药物的全身暴露,因此对正常细胞的非靶向毒性较低。可裂解连接子使其具有更强的 “旁观者”效应,同时增强了有效载荷的组织穿透能力。因此,T-DM1虽然与T-DXd具有相似的细胞效力,但后者对NCI-N87异种移植瘤小鼠的抑制作用更明显[7]。
Shitara等[8]的一项开放标签、随机、Ⅱ期研究发现,T-DXd组客观反应率(objective response rate,ORR)为51%,医生选择化疗组为14%(P<0.001)。同时,T-DXd组OS显著高于选择化疗组(中位数分别为12.5和8.4个月,死亡危险比为0.59,95% CI:0.39~0.80,P=0.01,超过了预先设定的奥布莱恩-弗莱明界限)。基于此,T-DXd被美国食品药品管理局(Food And Drug Administration,FDA)突破性疗法认定用于治疗HER2阳性晚期胃腺癌患者。由于四肽连接子更易于酶解,因此T-DXd的“旁观者”效应比T-DM1更强,能够抑制HER2低表达[免疫组织化学检测(immunohistochemistry,IHC)IHC+/++或荧光原位杂交(fluorescence in situ hybridization,FISH)阴性]和trastuzumab或T-DM1不敏感胃癌细胞的增殖。一项剂量递增和扩展Ⅰ期研究显示T-DXd在HER2低表达实体瘤患者中具有一定的治疗效果,所有入组患者(n=58)均接受T-DXd 6.4 mg/kg Q3W治疗,结果显示,患者ORR为37.0%(95% CI:24.3%~51.3%),应答持续时间中位值为10.4个月(95% CI:8.8个月~无法评估)[9]。因此,无论HER2表达高低,T-DXd均具有良好的活性和抗肿瘤作用。但是,HER2表达水平被证明可能会影响T-DXd的疗效。进一步研究[8]发现,T-DXd对HER2(IHC+++)胃癌患者的有效率高于HER2(IHC++和ISH+)低表达患者,其ORR分别为58%和29%。然而,由于该研究样本量较小,其结论尚存在一定争议。
DISTY-Gastric01(NCT03329690)是一项多中心、开放标签Ⅱ期临床研究,旨在评估DS-8201在HER2阳性胃癌或胃食管交界癌患者三线和后线治疗中的效果和安全性,该研究共纳入187例接受过至少两种方案治疗后的胃癌患者。其中,125例患者接受T-DXd治疗,62例患者接受化疗(55例患者接受伊立替康治疗,7例患者接受紫杉醇治疗)。结果显示,T-DXd组中位客观反应持续时间为11.3个月(95% CI:5.6~无法估计),选择化疗组为3.9个月(95% CI:3.0~4.9)。两组中位反应时间相似[T-DXd组1.5个月(95% CI:1.4~1.7),选择化疗组1.6个月(95% CI:1.3~1.7)]。安全性方面,3级或更高级别AE为中性粒细胞计数下降(T-DXd组为51%,选择化疗组为24%)、贫血(分别为38%和23%)和白细胞计数降低(分别为21%和11%)。有12例患者出现trastuzumab相关间质性肺病或肺炎,在T-DXd组中出现1例药物相关死亡[9]。总体而言,T-DXd显示出可控的安全性。DESTINY-Gastric02研究(NCT04014075)公布了与之相似的数据,该研究主要纳入含trastuzumab治疗后进展的HER2阳性胃癌或胃食管交界癌患者(n=79)。截至2021年4月9日,T-DXd组ORR为38%,疾病控制率(disease control rate,DCR)为81%,中位PFS为5.5个月,中位治疗持续时间为4.3个月。最常见致停药AE为肺炎(3.8%)和间质性肺病(2.5%),最常见的致药物减量AE为恶心(7.6%)和中性粒细胞计数减少(5.1%)[10]。该研究为T-DXd作为HER2阳性胃癌的三线及后线治疗提供了有力的临床依据,并为后期正在进行的Ⅲ期随机试验DESTINY-Gastric04(NCT04704934)提供了数据支持[11]。尽管T-DXd在治疗过程中患者存在骨髓抑制和间质性肺炎的风险。但是,由于其疗效优于T-DM1和可控的安全性,T-DXd成为首个用于治疗接受过至少两种或两种以上化疗方案HER2阳性晚期胃癌或胃食管交界癌患者的ADC。
1.3 维迪西妥单抗
维迪西妥单抗(RC48)是我国首创的ADC药物,由人源化hertuzumab通过可裂解马来多环丙戌基瓜氨酸对氨基苄氧羰基(MC-Val-Cit-PABC-PNP)与一甲基澳瑞他汀E(MMAE)连接组成。与trastuzumab相比,hertuzumab对HER2阳性细胞的亲和力更高,具有更强的ADCC效应。此外,RC48与肿瘤细胞的特异性结合不受MMAE结合的影响,尽管hertuzumab与MMAE结合能够降低ADCC效应,但总体而言对HER2阳性胃癌细胞仍有显著的细胞毒性[12]。更重要的是,当RC48与HER2过表达及HER2阴性胃癌细胞共培养时,RC48对HER2阴性细胞也具有杀灭效应,且3.3 mg/kg剂量RC48较10 mg/kg剂量T-DM1具有更强的“旁观者效应”,这可能与缬氨酸-瓜氨酸(valine-citrulline,vc)连接子有关。vc连接子通过利用肿瘤微环境和正常细胞内的pH值差异来释放有效载荷,可显著提高ADC的治疗指数和靶向性[13]。因此,RC48在到达肿瘤细胞后vc连接子被组织蛋白酶B切割降解,从而释放有效载荷杀伤肿瘤细胞。而正常细胞中由于pH值较高且相对缺乏组织蛋白酶B,vc连接子无法在体循环中降解,故降低了其非靶向毒性[14- 16]。此外,RC48能够显著抑制HER2阳性胃癌细胞增殖,呈剂量依赖性,该效应也适用于一些耐药细胞株[17]。Chen等[16]通过体内实验发现,与T-DM1相比RC48对胃癌细胞以及在trastuzumab和拉帕替尼耐药的异种移植瘤模型中具有更强的抗肿瘤活性,这与RC48有效载荷在扩散到邻近非靶向癌细胞时发挥的“旁观者” 效应有关,提示其作为HER2阳性胃癌改良疗法的潜力。Li等[18]在NCI-N87细胞构建的小鼠人源肿瘤细胞系异种移植(cell-derived xenograft,CDX)模型中发现,RC48的抗肿瘤活性比trastuzumab、hertuzumab、MMAE单一治疗以及hertuzumab联合MMAE的治疗效果更强。
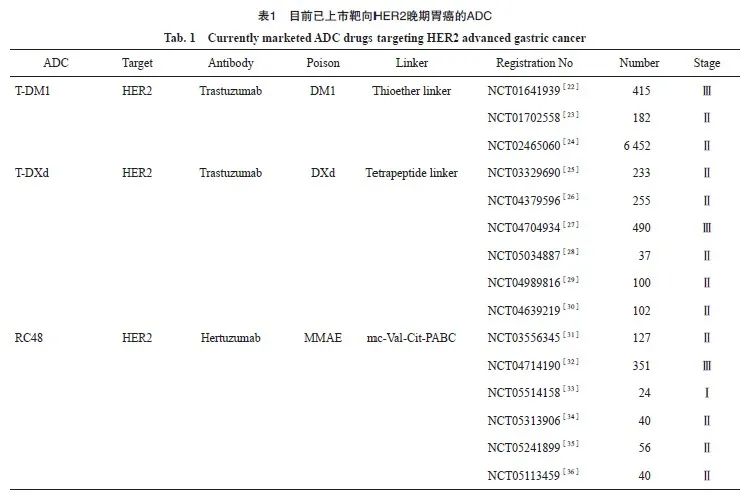
RC48对HER2低表达胃癌同样具有抑制效应。金洋冰等[19]使用RC48处理HER2低表达细胞系后发现,RC48对MGC803细胞48及72 h的半数抑制浓度(IC50)分别为9.449及6.056 μg/mL,其他几种细胞(MKN7、MKN45、HGC27)48 h的IC50分别为 24.20、331.90及35.32 μg/mL,72 h的IC50分别为66.48、63.44及26.43 μg/mL。此外,部分HER2低表达患者在接受RC48治疗后达到部分缓解(partial response,PR)。一项在晚期或转移性胃癌或胃食管交界癌患者中的Ⅱ期研究(NCT03556345)[20]也显示出相似的结果,患者ORR为24.8%,中位PFS为4.1个月,中位生存期为7.9个月。值得注意的是,HER2低表达患者约占所有胃癌患者的40%~60%,这也进一步扩大了RC48的治疗适应证。Xu等[21]发现,RC48对HER2 IHC++/FISH-患者的反应(ORR为35.7%)与IHC++/FISH+(ORR为20%)和IHC+++(ORR为13.6%)患者相似。因此, RC48对HER2阳性胃癌患者具有显著的抗肿瘤活性和可耐受的安全性,且该效应不完全依赖于HER2表达高低。同时RC48联合trastuzumab、紫杉醇等药物可能是治疗进展期HER2阳性胃癌的一种有前途的治疗策略,有望在未来取得更多可喜的结果。目前已上市靶向HER2晚期胃癌的ADC见表1。
2 ADC在HER2阳性晚期胃癌中的最新研究
除上述几种已上市用于晚期胃癌治疗的ADC外,目前全球在研治疗晚期胃癌的靶向HER2 ADC多处于临床前研究阶段,且疗效和安全性尚不完全清楚。
2.1 SYD985
SYD985是一种新型ADC,由trastuzumab、可裂解vc-塞科-杜卡霉素-羟基苯甲酰胺-氮杂吲哚(vc-seco-DUBA)连接子连接杜卡霉素衍生物组成,DAR为2.8。SYD985被内吞进入靶细胞后,vc-seco-DUBA被溶酶体内蛋白酶水解进而激活释放高活性的杜卡霉素,通过与DNA小沟的鸟嘌呤残基结合,使DNA发生不可逆性烷基化,最终杀伤肿瘤细胞。此外,杜卡霉素膜通透性较高,能够有效诱导“旁观者”效应,杀伤非靶向肿瘤细胞。
在临床前研究中,Nadal-serrano等[37]发现SYD985和T-DM1在效应细胞的激发下,能够对HER2低表达细胞系诱导出相似水平的ADCC作用。此外,SYD985能够克服对T-DM1耐药细胞的耐药性。与T-DM1不同,SYD985具有可被溶酶体蛋白酶和膜渗透性药物切割的连接子,介导 “旁观者”效应,消除邻近HER2表达阴性癌细胞[38-39]。基于上述研究,Banerji等[39]对其进行了Ⅰ期剂量递增研究,以评估SYD985的安全性,随后进行了Ⅰ期剂量扩展研究,以评价其在HER2阳性晚期胃癌患者中的活性。在剂量递增队列中的16例胃癌患者中,1例(6%,95% CI:0.2~30.2)达到PR,中位PFS(median PFS,mPFS)为3.2个月(95% CI:1.6~5.3)。同时, Ⅰ阶段临床研究(NCT02512237)结果证实了SYD985的抗肿瘤活性,尽管其具有明显的眼部不良反应[39-40]。另一项SYD985与紫杉醇联合治疗晚期胃癌的开放标签、单臂、多位点Ⅰ/Ⅰb期研究(NCT04602117)正在进行,旨在评估SYD985联合紫杉醇在晚期胃癌中的治疗效果。
2.2 ARX788
ARX788由trastuzumab与微管蛋白抑制剂amberstatin(AS269)组成,DAR为2。ARX-788通过在人源化抗HER2抗体两条重链上的优化位置引入合成氨基酸,从而实现了位点的特异性结合。ARX788在进入肿瘤细胞后,通过释放微管蛋白抑制剂amberstatin,抑制细胞有丝分裂,诱导细胞周期停滞、死亡,从而发挥抗癌活性。
ARX788能够抑制HER2阳性肿瘤细胞的生长。此外,AX788可以杀伤抗T-DM1胃癌细胞[41]。Barok等[41]发现,ARX788能显著抑制胃癌细胞增殖,尤其对T-DM1耐药的ROE-19和RN-87细胞系,且呈剂量依赖性。同时,ARX788在4种胃癌细胞系(OE-19、ROE-19、N-87、RN-87)中均达到IC50,而T-DM1仅在OE-19和N-87细胞中达到IC50。在这些细胞系中,ARX788较T-DM1相比分别产生了1.35倍(OE-19)和3.6倍(N-87)的效力,提示在HER2阳性胃癌中,尤其对T-DM1耐药胃癌的治疗,ARX788比T-DM1更有效。值得注意的是,ARX788在正常心肌细胞中无活性。小鼠人源肿瘤组织来源移植瘤(patientl-derived xenograft,PDX)模型也证实ARX788在不同表达水平HER2阳性肿瘤以及T-DM1耐药模型中均具有较强的抗肿瘤活性,且耐受性良好,未观察到明显的体重减轻[41]。一项Ⅰ期多中心剂量扩展研究(CTR20190639)[42]显示,ARX788的确诊ORR为37.9%,mPFS和中位OS(median OS,mOS)分别为4.1和10.7个月。只有13.3%的患者发生了与ARX788相关的3级AE。因此,ARX788在转移性HER2阳性胃癌或胃食管交界癌患者中具有良好的耐受性和抗癌活性。基于上述结果,ARX788于2021年1月被FDA授予胃癌孤儿药资格,用于HER2阳性胃癌或胃食管交界癌患者的二线治疗。目前,针对ARX788在HER2阳性胃癌或胃食管交界癌患者的全球(ACE-Gastric-02)Ⅱ/Ⅲ期临床试验正在开展。
2.3 MRG002
MRG002由糖修饰化trastuzumab通过可酶切vc连接子与MMAE偶联而成,DAR为3.8。由于其抗体部分高度岩藻糖基化,因此ADCC活性较低,对免疫细胞的影响较小。MMAE是一种广泛应用于ADC的微管蛋白抑制剂,可抑制微管蛋白聚合来干扰有丝分裂。vc连接子稳定性良好,且更易诱导“旁观者”效应。同时,其完整性在96 h后仍高达76%。MRG002与HER2蛋白特异性结合后,通过内化、转位在溶酶体中发生裂解,快速释放MMAE,进而杀伤肿瘤细胞[43]。Li等[43]发现,MRG002在不同表达水平HER2阳性胃癌异种移植瘤模型中显示出显著的抗肿瘤活性。在HER2阳性胃癌细胞(NCI-N87)中观察到,MRG002在亚毫摩尔浓度下就能发挥显著的细胞毒性。此外,在所有HER2阳性细胞中,MRG002的细胞毒性均强于恩美曲妥珠单抗。在HER2高表达乳腺癌细胞系(BT-474)和胃癌细胞系(NCI-N87)构建的CDX模型,MRG002在0.3 mg/kg时就能够显著抑制肿瘤细胞生长,而对照药物恩美曲妥珠单抗在两种模型中均为 3.0 mg/kg时才有效。随后对11个PDX模型(包括8个胃癌模型,3个乳腺癌模型,其中8个为trastuzumab耐药)研究发现,MRG002单一治疗可显著抑制11个PDX模型中的肿瘤生长,其抗肿瘤活性始终高于同等剂量下的恩美曲妥珠单抗。尤其是MRG002在恩美曲妥珠单抗耐药的胃PDX模型中显示出显著的肿瘤抑制活性。此外,MRG002与抗程序性死亡[蛋白]-1 (programmed death-1,PD-1)mAb HX008联合在相同剂量下显示出比单独使用MRG002或HX008更高的抗肿瘤活性,提示MRG002与抗PD-1 mAb组合能够显着增强抗肿瘤细胞活性。
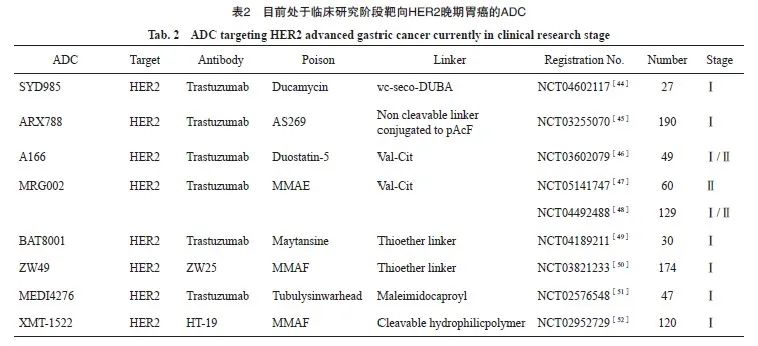
总之,上述结果表明,MRG002对HER2阳性胃癌治疗有效,尤其对于恩美曲妥珠单抗耐药细胞。同时,MRG002治疗HER2阳性低表达局部晚期或转移性胃癌或胃食管交界癌的临床研究(NCT05141747)正在进行,有望为HER2低表达胃癌患者的治疗带来新希望。目前处于临床研究阶段靶向HER2晚期胃癌的ADC见表2。
3 ADC联合ICI在HER2晚期胃癌中的研究进展
免疫治疗是一种新兴的肿瘤治疗模式。在晚期胃癌中,免疫治疗的策略主要包括过继细胞疗法、肿瘤疫苗和免疫检查点的调节等。近年来,在晚期胃癌免疫治疗领域,特别是靶向免疫检查点治疗的发展突飞猛进。CheckMate 649研究[53]奠定了ICI在晚期胃癌免疫治疗中的重要地位,2022年欧洲肿瘤内科学会(European Society for Medical Oncology,ESMO)大会更新了最新的研究结果,进一步确认了纳武利尤单抗联合化疗在全人群中的效果,所有随机患者ORR由46%升至58%,PFS和OS均得到延长,且安全性良好。该随访结果进一步支持纳武利尤单抗联合化疗作为晚期胃癌患者的一线标准治疗。在Ⅲ期KEYNOTE 062试验[54]中,PD-1抑制剂pembrolizumab单独或与化疗联合作为晚期胃癌一线治疗药物时,pembrolizumab疗效不逊于化疗,且AE发生更少。PD-1抑制剂也有利于HER2阳性胃癌的治疗。在Ⅲ期KEYNOTE-811研究[55]中,在标准trastuzumab联合化疗的基础上加入pembrolizumab治疗HER2阳性胃癌或胃食管交界癌后发现,trastuzumab+化疗联合pembrolizumab显著缩小了肿瘤大小,并显著提高了患者的ORR。该研究是继ToGA研究之后又一重大突破,改写了HER2阳性胃癌一线治疗指南,开启了HER2阳性胃癌“免疫治疗+靶向治疗+化疗”治疗新时代。在此基础上,对新型抗HER2药物及联合方式也展开了一系列探索。ZW25是一种靶向HER2的ECD4和ECD2结构域的双特异性抗体,同样在HER2阳性胃癌患者中与PD-1抑制剂和化疗的联合治疗下达到了与KEYNOTE-811相近的ORR及中位PFS为10.9个月的治疗效果[56]。此外,细胞毒性T淋巴细胞相关抗原4(cytotoxic T lymphocyte associated antigen-4,CTLA-4)是另一个重要的检查点。然而,在胃癌中CTLA-4检查点抑制剂尚未取得成功。目前正在尝试开发的PD-1/CTLA-4双靶点抑制剂,cadonilimab(AK104)是首个PD-1/CTLA-4双特异性抗体。在一项Ⅰb/Ⅱ期研究中,AK104与化疗联合用于胃癌或胃食管交界癌的一线治疗(NCT03852251),AK104显示出良好的抗癌活性和可控的安全性。AK104联合化疗作为胃癌或胃食管交界癌一线治疗的Ⅲ期研究(NCT05008783)正在进行中。
ADC有助于提高胃癌组织中T淋巴细胞浸润程度,增强抗肿瘤免疫反应,进而提高PD-1抑制剂的治疗效果。因此,一种有前途的治疗策略就是ADC联合ICI。ADC增强抗肿瘤免疫反应体现在以下3个方面:首先,ADC可在癌细胞内释放细胞毒性有效载荷诱导产生抗肿瘤免疫活性[57]。其次,ADC通过在肿瘤表面富集,进而对癌细胞或非靶向癌细胞产生直接杀伤作用,显著提高免疫反应。再次,细胞毒性有效载荷能够诱导抗原呈递树突状细胞成熟来增强抗肿瘤免疫反应。目前一些临床前研究已经证实了ADC联合ICI在胃癌治疗中的潜力,Traore等[58]使用XMT-1522处理多个胃癌细胞系后发现其可在体外诱导免疫细胞死亡,并增强CD8+T细胞浸润和CD8+细胞PD-1表达水平。Iwata等[59]发现, T-DXd可提高肿瘤浸润树突状细胞数量并上调其CD86表达。此外,T-DXd还可增加肿瘤浸润性CD8+T细胞的数量,并增强肿瘤细胞上程序性死亡[蛋白]配体-1(programmed death ligand-1,PD-L1)和主要组织相容性复合体(major histocompatibility complex,MHC)Ⅰ类分子的表达水平。当与ICI联合时,XMT-1522和T-DXd均显示出协同抗肿瘤活性。因此,ADC与ICI组成的联合疗法展现出“1+1>2”的抗癌效果,有望成为HER2阳性胃癌一种有前途的治疗策略,但需要大型随机对照临床研究来证实。
4 ADC在应用和研发过程中面临的挑战
作为目前发展最快的HER2靶向药物,ADC在应用和研发过程中主要面临三大挑战:首先,如何提高肿瘤细胞对ADC的摄取是其研发的主要困难。ADC主要依靠靶抗原在癌细胞表面的高表达来保证其发挥有效的内吞作用进而释放细胞毒性有效载荷。HER2 ADC的杀伤效应与胃癌细胞表面HER2表达水平密切相关,并且需要HER2在胃癌细胞表面高表达。但是,部分HER2低表达胃癌限制了现有ADC的治疗效果。因此,改善ADC的癌细胞摄取能显著提高其抗癌活性,尤其对于HER2低表达胃癌[60]。其次,非靶向毒性是导致ADC临床试验失败的主要因素之一。非靶向毒性与多种因素有关,包括mAb、细胞毒性有效载荷、连接子和靶抗原以及癌细胞对ADC的内化作用,抗体与Fc受体的非特异性结合,连接子过早水解以释放有效载荷,过强的ADCC作用引起的“旁观者”效应对正常细胞均可产生非靶向毒性作用[61]。再次,靶抗原低表达也是ADC脱靶毒性产生的另一个重要因素。ADC耐药是另一个需要被攻克的难题。研究[62]表明, ADC的无效内化和溶酶体降解可能是T-DM1失活的主要机制。通过上调药物外排泵或交替使用微管蛋白/微管相关蛋白也可能是癌细胞对T-DM1产生耐药的原因。此外,trastuzumab相关改变也可能促成T-DM1耐药的发生,包括 HER2表达水平降低、HER2截短形式的出现或ERBB2基因突变[66]。同时,一些关键问题也应该在未来得到解决,包括优化每个ADC组分以提高其抗癌活性;加快探索除HER2之外的其他靶点,如针对Trop-2[64]、c-MET[65]、claudin 18.2[66]和鸟苷酸环化酶C(guanylate cyclase C,GCC) [67]的ADC等。
5 总结与展望
尽管在ToGA研究中,trastuzumab联合其他化疗药物治疗HER2阳性晚期胃癌的疗效优于单独化疗,但随后针对晚期HER2阳性胃癌的诸多相关研究均以失败告终。部分mAb、小分子酪氨酸激酶抑制剂等在HER2阳性晚期胃癌中均未取得令人满意的结果,因此在HER2阳性胃癌的治疗方面仍面临巨大挑战。ADC将mAb与化疗药物的优点完美结合,以较低的全身毒性将治疗量的细胞毒性药物靶向递送至肿瘤细胞内,实现了更宽的治疗窗口和更强的药代动力学特性。凭借这一显著优势,ADC在HER2阳性晚期胃癌的治疗方面显示出极大的潜力。但ADC在应用和研发过程中也面临耐药、药物提前降解、非靶向毒性作用等一系列挑战。
总之,ADC是有效且耐受性良好的新型抗肿瘤药物,其快速发展是肿瘤免疫治疗的又一大突破,也是HER2阳性晚期胃癌未来治疗的希望所在。随着定点偶联技术的完善、新型有效载荷的发现以及抗体修饰技术的不断进步,ADC类药物必将日趋完善,并在临床实践中发挥显著的抗癌功效。
利益冲突声明:所有作者均声明不存在利益冲突。
[参考文献]
[1] LE GALL C M, VAN DER SCHOOT J M S, RAMOSTOMILLERO I, et al. Dual site-specific chemoenzymatic antibody fragment conjugation using CRISPR-based hybridoma engineering[J]. Bioconjug Chem, 2021, 32(2): 301-310.
[2] MAMOUNAS E P, UNTCH M, MANO M S, et al. Adjuvant T-DM1 versus trastuzumab in patients with residual invasive disease after neoadjuvant therapy for HER2-positive breast cancer: subgroup analyses from KATHERINE[J]. Ann Oncol, 2021, 32(8): 1005-1014.
[3] ZHANG J H, FAN J J, ZENG X, et al. Targeting the autophagy promoted antitumor effect of T-DM1 on HER2-positive gastric cancer[J]. Cell Death Dis, 2021, 12(4): 288.
[4] VON MINCKWITZ G, HUANG C S, MANO M S, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer[J]. N Engl J Med, 2019, 380(7): 617-628.
[5] 《中国乳腺癌新辅助治疗专家共识(2022年版)》专家组. 中国乳腺癌新辅助治疗专家共识(2022年版)[J]. 中国癌症杂志, 2022, 32(1): 80-89.
Expert Group of Expert Consensus on Neoadjuvant Treatment of Breast Cancer in China (2022 edition). Expert consensus on neoadjuvant treatment of breast cancer in China (2022 edition)[J]. China Oncol, 2022, 32(1): 80-89.
[6] SHITARA K, HONMA Y, OMURO Y, et al. Efficacy of trastuzumab emtansine in Japanese patients with previously treated HER2-positive locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma: a subgroup analysis of the GATSBY study[J]. Asia Pac J Clin Oncol, 2020, 16(1): 5-13.
[7] OGITANI Y, AIDA T, HAGIHARA K, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase Ⅰ inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1[J]. Clin Cancer Res, 2016, 22(20): 5097-5108.
[8] SHITARA K, BANG Y J, IWASA S, et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer[J]. N Engl J Med, 2020, 382(25): 2419-2430.
[9] MOD I, PARK H, MURTHY R K, et al. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-lowexpressing advanced breast cancer: results from a phase Ⅰb study[J]. J Clin Oncol, 2020, 38(17): 1887-1896.
[10] KALMUK J, RINDER D, HELTZEL C, et al. An overview of the preclinical discovery and development of trastuzumab deruxtecan: a novel gastric cancer therapeutic[J]. Expert Opin Drug Discov, 2022, 17(5): 427-436.
[11] AOKI M, IWASA S, BOKU N. Trastuzumab deruxtecan for the treatment of HER2-positive advanced gastric cancer: a clinical perspective[J]. Gastric Cancer, 2021, 24(3): 567-576.
[12] LI L, XU M Z, WANG L, et al. Conjugating MMAE to a novel anti-HER2 antibody for selective targeted delivery[J]. Eur Rev Med Pharmacol Sci, 2020, 24(24): 12929-12937.
[13] HA S Y Y, ANAMI Y, YAMAZAKI C M, et al. An enzymatically cleavable tripeptide linker for maximizing the therapeutic index of antibody-drug conjugates[J]. Mol Cancer Ther, 2022, 21(9): 1449-1461.
[14] 季双敏, 王玉珠, 杨进波. 抗体偶联药物的分子特点及其药代动力学研究考虑[J]. 中国临床药理学杂志, 2021, 37(6): 777-782.
JI S M, WANG Y Z, YANG J B. Molecular characteristics and pharmacokinetic considerations of antibody coupled drugs[J]. Chin J Clin Pharmacol, 2021, 37(6): 777-782
[15] BURKE J M, MORSCHHAUSER F, ANDORSKY D, et al. Antibody-drug conjugates for previously treated aggressive lymphomas: focus on polatuzumab vedotin[J]. Expert Rev Clin Pharmacol, 2020, 13(10): 1073-1083.
[16] CHEN Z H, YUAN J J, XU Y Y, et al. From AVATAR mice to patients: RC48-ADC exerted promising efficacy in advanced gastric cancer with HER2 expression[J]. Front Pharmacol, 2021, 12: 757994.
[17] HU Y X, ZHU Y X, WEI X W, et al. Disitamab vedotin, a novel HER2-directed antibody-drug conjugate in gastric cancer and other solid tumors[J]. Drugs Today (Barc), 2022, 58(10): 491-507.
[18] LI H W, YU C, JIANG J, et al. An anti-HER2 antibody conjugated with monomethyl auristatin E is highly effective in HER2-positive human gastric cancer[J]. Cancer Biol Ther, 2016, 17(4): 346-354.
[19] 金洋冰, 蔡 劬, 计 骏, 等. 抗体偶联药物维迪西妥单抗对HER-2不同表达水平胃癌细胞抑制效应的体外研究[J].临床肿瘤学杂志, 2023, 28(1): 1-7.
JIN Y B, CAI Q, JI J, et al. In vitro study on the inhibitory effect of antibody coupled drug disitamab vedotin on gastric cancer cells with different expression levels of HER-2[J]. Chin Clin Oncol, 2023, 28(1): 1-7.
[20] PENG Z, LIU T S, WEI J, et al. Efficacy and safety of a novel anti-HER2 therapeutic antibody RC48 in patients with HER2-overexpressing, locally advanced or metastatic gastric or gastroesophageal junction cancer: a single-arm phase Ⅱ study[J]. Cancer Commun (Lond), 2021, 41(11): 1173-1182.
[21] XU Y Y, WANG Y K, GONG J F, et al. Phase Ⅰ study of the recombinant humanized anti-HER2 monoclonal antibody-MMAE conjugate RC48-ADC in patients with HER2-positive advanced solid tumors[J]. Gastric Cancer, 2021, 24(4): 913-925.
[22] ClinicalTrials.gov. A study of trastuzumab emtansine versus taxane in participants with human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer[EB/OL]. [2022-10-25]. https://clinicaltrials.gov/ct2/show/NCT01641939.
[23] ClinicalTrials.gov. A combination study of Kadcyla (trastuzumab emtansine) and capecitabine in participants with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (mBC) or HER2-positive locally advanced/metastatic gastric cancer (LA/mGC) (TRAXHER2) [EB/OL]. [2022-10-25]. https://clinicaltrials.gov/ct2/show/NCT01702558.
[24] ClinicalTrials.gov. Targeted therapy directed by genetic testing in treating patients with advanced refractory solid tumors, lymphomas, or multiple myeloma (the MATCH screening trial)[EB/OL]. [2022-10-25]. https://clinicaltrials.gov/ct2/show/NCT02465060.
[25] ClinicalTrials.gov. DS-8201a in human epidermal growth factor receptor 2 (HER2)-expressing gastric cancer [DESTINYGastric01][EB/OL]. [2022-10-25].https://clinicaltrials.gov/ct2/show/NCT03329690.
[26] ClinicalTrials.gov. Ph1b/2 study of the safety and efficacy of T-DXd combinations in advanced HER2+ gastric cancer (DESTINY-Gastric03) (DG-03) [EB/OL]. [2022-10-25]. https://clinicaltrials.gov/ct2/show/NCT04379596.
[27] ClinicalTrials.gov. Trastuzumab deruxtecan for subjects with HER2-positive gastric cancer or gastro-esophageal junction adenocarcinoma after progression on or after a trastuzumab-containing regimen (DESTINY-Gastric04) [EB/OL]. [2022-10-25].https://clinicaltrials.gov/ct2/show/
NCT04704934.
[28] ClinicalTrials.gov. Phase 2 study of T-DXd in the neoadjuvant treatment for patients with HER2 positive gastric and GEJ adenocarcinoma [EB/OL]. [2022-10-25].https://clinicaltrials.gov/ct2/show/NCT05034887.
[29] ClinicalTrials.gov. Study of T-DXd monotherapy in patients with HER2-expressing locally advanced or metastatic gastric or GEJ adenocarcinoma who have received 2 or more prior regimens (DG-06)[EB/OL]. [2022-10-25]. https://clinicaltrials.gov/ct2/show/NCT04989816.
[30] ClinicalTrials.gov. A study of T-DXd for the treatment of solid tumors harboring HER2 activating mutations (DPT01) [EB/OL]. [2022-10-25]. https://clinicaltrials.gov/ct2/show/NCT04639219.
[31] ClinicalTrials.gov. A study of RC48-ADC in local advanced or metastatic gastric cancer subjects with the overexpression of HER2 [EB/OL]. [2022-10-25]. https://clinicaltrials.gov/ct2/show/NCT03556345.
[32] ClinicalTrials.gov. A study of RC48-ADC in local advanced or metastatic gastric cancer with the HER2-overexpression[EB/OL]. [2022-10-25]. https://clinicaltrials.gov/ct2/show/NCT04714190.
[33] ClinicalTrials.gov. To evaluate the safety, tolerability, pharmacokinetics and preliminary efficacy of disitamab vedotin combined with RC98 in the treatment of subjects with HER2- expressing locally advanced or metastatic gastric cancer (including AEG) [EB/OL]. [2022-10-25]. https://clinicaltrials.gov/ct2/show/NCT05514158.
[34] ClinicalTrials.gov. RC48 plus AK105 and cisplatin in advanced gastric cancer [EB/OL]. [2022-10-25]. https://clinicaltrials.gov/ct2/show/NCT05313906.
[35] ClinicalTrials.gov. A phase Ⅱ clinical study of fruquintinib combined with RC48 in the treatment of previously treated HER2-positive locally advanced or metastatic gastric or gastroesophageal junction (G/GEJ) cancer [EB/OL].[2022-10-25]. https://clinicaltrials.gov/ct2/show/NCT05241899.
[36] ClinicalTrials.gov. Disitamab vedotin combined with PD-1 and neoadjuvant chemotherapy for locally advanced gastric cancer(RC48-C018)[EB/OL]. [2022-10-25]. https://clinicaltrials.gov/ct2/show/NCT05113459.
[37] NADAL-SERRANO M, MORANCHO B, ESCRIVÁ-DEROMANÍ S, et al. The second generation antibody-drug conjugate SYD985 overcomes resistances to T-DM1[J]. Cancers (Basel), 2020, 12(3): 670.
[38] DURO-SÁNCHEZ S, NADAL-SERRANO M, LALINDEGUTIÉRREZ M, et al. Therapy-induced senescence enhances the efficacy of HER2-targeted antibody-drug conjugates in breast cancer[J]. Cancer Res, 2022, 82(24): 4670-4679.
[39] BANERJI U, VAN HERPEN C M L, SAURA C, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study[J]. Lancet Oncol, 2019, 20(8): 1124-1135.
[40] MENDERES G, BONAZZOLI E, BELLONE S, et al. SYD985, a novel duocarmycin-based HER2-targeting antibody-drug conjugate, shows promising antitumor activity in epithelial ovarian carcinoma with HER2/Neu expression[J]. Gynecol Oncol, 2017, 146(1): 179-186.
[41] BAROK M, LE JONCOUR V, MARTINS A, et al. ARX788, a novel anti-HER2 antibody-drug conjugate, shows anti-tumor effects in preclinical models of trastuzumab emtansine-resistant HER2-positive breast cancer and gastric cancer[J]. Cancer Lett, 2020, 473: 156-163.
[42] ZHANG J, JI D M, SHEN W N, et al. Phase Ⅰ trial of a novel anti-HER2 antibody-drug conjugate, ARX788, for the treatment of HER2-positive metastatic breast cancer[J]. Clin Cancer Res, 2022. Online ahead of print.
[43] LI H, ZHANG X, XU Z Y, et al. Preclinical evaluation of MRG002, a novel HER2-targeting antibody-drug conjugate with potent antitumor activity against HER2-positive solid tumors[J]. Antib Ther, 2021, 4(3): 175-184.
[44] ClinicalTrials.gov. ISPY-P1.01: evaluating the safety of weekly paclitaxel with trastuzumab duocarmazine (SYD985) in patients with metastatic cancer [EB/OL]. [2022-10-25]. https://clinicaltrials.gov/ct2/show/NCT04602117.
[45] ClinicalTrials.gov. A dose-escalation, expansion study of ARX788, in advanced solid tumors subjects with HER2 expression (ACE-Pan Tumor 01) [EB/OL]. [2022-10-25]. https://clinicaltrials.gov/ct2/show/NCT03255070.
[46] ClinicalTrials.gov. Study of A166 in patients with relapsed/refractory cancers expressing HER2 antigen or having amplified HER2 gene [EB/OL]. [2022-10-25]. https://clinicaltrials.gov/ct2/show/NCT03602079.
[47] ClinicalTrials.gov. A study of MRG002 in the treatment of HER2-positive/HER2-low locally advanced or metastatic gastric/gastroesophageal junction cancer. [EB/OL]. [2022-10-25]. https://clinicaltrials.gov/ct2/show/NCT05141747.
[48] ClinicalTrials.gov. A study of MRG002 in patients with HER2-positive advanced solid tumors and locally advanced or metastatic gastric/gastroesophageal junction (GEJ) cancer [EB/OL]. [2022-10-25]. https://clinicaltrials.gov/ct2/show/NCT04492488.
[49] ClinicalTrials.gov. A clinical trial of BAT8001 on safety, tolerability and pharmacokinetics for patients [EB/OL].[2022-10-25]. https://clinicaltrials.gov/ct2/show/NCT04189211.
[50] ClinicalTrials.gov. A dose finding study of ZW49 in patients with HER2-positive cancers [EB/OL]. [2022-10-25]. https://clinicaltrials.gov/ct2/show/NCT03821233.
[51] ClinicalTrials.gov. A phase 1/2 study of MEDI4276 in adults subjects with select HER2-expressing advanced solid tumors. (MEDI4276) [EB/OL]. [2022-10-25]. https://clinicaltrials.gov/ct2/show/NCT02576548.
[52] ClinicalTrials.gov. Study of antibody drug conjugate in patients with advanced breast cancer expressing HER2 [EB/OL]. [2022-10-25]. https://clinicaltrials.gov/ct2/show/NCT02952729.
[53] JANJIGIAN Y Y, SHITARA K, MOEHLER M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial[J]. Lancet, 2021, 398(10294): 27-40.
[54] SHITARA K, VAN CUTSEM E, BANG Y J, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial[J]. JAMA Oncol, 2020, 6(10): 1571-1580.
[55] JANJIGIAN Y Y, KAWAZOE A, YAÑEZ P, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer[J]. Nature, 2021, 600(7890): 727-730.
[56] MERIC-BERNSTAM F, BEERAM M, HAMILTON E, et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2- amplified cancers: a phase 1, dose-escalation and expansion study[J]. Lancet Oncol, 2022, 23(12): 1558-1570.
[57] DELA CRUZ CHUH J, GO M, CHEN Y, et al. Preclinical optimization of Ly6E-targeted ADCs for increased durability and efficacy of anti-tumor response[J]. MAbs, 2021, 13(1): 1862452.
[58] TRAORE T, KHATTAR M. Synergy of an anti-HER2 ADC TAK-522 (XMT-1522) in combination with anti-PD1 monoclonal antibody (mAb) in a syngeneic breast cancer model expressing human HER2[J]. Cancer Res, 2018, 78(13_Suppl): LB-294.
[59] IWATA T N, ISHII C, ISHIDA S, et al. A HER2-targeting antibody-drug conjugate, trastuzumab deruxtecan (DS-8201a), enhances antitumor immunity in a mouse model[J]. Mol Cancer Ther, 2018, 17(7): 1494-1503.
[60] YU M H, LIANG Y, LI L H, et al. Research progress of antibody-drug conjugates therapy for HER2-low expressing gastric cancer[J]. Transl Oncol, 2023, 29: 101624.
[61] SIMMONS J K, BURKE P J, COCHRAN J H, et al. Reducing the antigen-independent toxicity of antibody-drug conjugates by minimizing their non-specific clearance through PEGylation[J]. Toxicol Appl Pharmacol, 2020, 392: 114932.
[62] DÍAZ-RODRÍGUEZ E, GANDULLO-SÁNCHEZ L, OCAÑA A, et al. Novel ADCs and strategies to overcome resistance to anti-HER2 ADCs[J]. Cancers (Basel), 2021, 14(1): 154.
[63] GINZEL J D, ACHARYA C R, LUBKOV V, et al. HER2 isoforms uniquely program intratumor heterogeneity and predetermine breast cancer trajectories during the occult tumorigenic phase[J]. Mol Cancer Res, 2021, 19(10): 1699-1711.
[64] TARANTINO P, CARMAGNANI PESTANA R, CORTI C, et al. Antibody-drug conjugates: smart chemotherapy delivery across tumor histologies[J]. CA Cancer J Clin, 2022, 72(2): 165-182.
[65] 候涛涛, 张剑锋, 雷世睿, 等. HGF、c-Met及p-Erk蛋白在胃癌组织中的表达及意义[J]. 现代肿瘤医学, 2021, 29(5): 819-823.
HOU T T, ZHANG J F, LEI S R, et al. Expression and significance of HGF, c-Met and p-Erk in gastric cancer tissues[J]. J Mod Oncol, 2021, 29(5): 819-823.
[66] 王俏丽, 杜 娟. CLDN18.2在消化系统恶性肿瘤中作用的研究进展[J]. 中国肿瘤生物治疗杂志, 2022, 29(7): 681-685.
WANG Q L, DU J. Research progress on the role of CLDN18.2 in malignant tumors of the digestive system [J]. Chin J Tumor Biother, 2022, 29(7): 681-685.
[67] BOSE A, BANERJEE S, VISWESWARIAH S S. Mutational landscape of receptor guanylyl cyclase C: functional analysis and disease-related mutations[J]. IUBMB Life, 2020, 72(6): 1145-1159.


