【论著】| RSPO3抑制体内结直肠癌移植瘤生长和增加NK细胞浸润的作用研究
时间:2023-08-27 15:43:32 热度:37.1℃ 作者:网络
[摘要] 背景与目的:结直肠癌的发生、发展涉及多个癌基因的激活和抑癌基因的失活,野生型R-脊椎蛋白3(R-spondin 3,RSPO3)在结直肠癌生长中的作用目前尚不清楚,本研究旨在探讨RSPO3对结直肠癌生长的影响并探索其潜在机制。方法:采用生物信息学分析RSPO3在结直肠癌及泛癌组织中的表达,分析结直肠癌中RSPO3表达与自然杀伤(natural killer,NK)细胞浸润、NK细胞激活分子表达的相关性。利用短发夹RNA(short hairpin RNA,shRNA)和慢病毒感染建立RSPO3敲减的SW480-RSPO3-KD细胞株、RSPO3过表达的HCT116-RSPO3-OE细胞株及相应的对照细胞株。采用细胞计数试剂盒-8(cell counting kit-8,CCK-8)检测体外各稳定转染细胞株的细胞增殖。采用流式细胞术分析各稳定转染细胞株的细胞周期、裸小鼠脾脏和移植瘤组织中NK细胞的比例。通过裸小鼠皮下移植瘤模型观察RSPO3敲减或过表达的结肠癌细胞在裸小鼠体内的生长。利用双荧光素酶报告基因系统检测RSPO3敲减或过表达对结肠癌Wnt基因转录活性的影响。结果:生物信息学分析显示,RSPO3在多种实体瘤肿瘤组织包括结直肠癌组织中的表达显著低于相应的癌旁组织。RSPO3敲减或过表达不影响体外SW480和HCT116结肠癌细胞的增殖(P>0.05)和细胞周期(P>0.05)。但在裸小鼠体内,与对照细胞相比,RSPO3敲减显著促进SW480细胞移植瘤的生长(260.2±162.4 vs 1 311.7±570.1,P<0.05),而RSPO3过表达则显著抑制HCT116细胞移植瘤的生长(1 549.0±241.2 vs 512.1±250.0,P<0.05)。流式细胞术分析发现,在荷移植瘤裸小鼠体内,RSPO3敲减显著减少了脾脏和移植瘤组织中NK细胞的比例(脾脏:6.42±0.94 vs 5.25±0.59,P=0.04;移植瘤:8.27±0.29 vs 6.48±1.48,P=0.04);而RSPO3过表达显著增加了脾脏和移植瘤组织中NK细胞的比例(脾脏:5.29±0.16 vs 7.02±0.49,P=0.01;移植瘤:6.39±0.39 vs 8.14±0.34,P<0.05)。癌症基因组图谱(The Cancer Genome Atlas,TCGA)数据相关性分析显示,RSPO3表达与NK细胞表面标志物CD56(r=0.58,P<0.05)和CD16(r=0.64,P<0.05)的表达显著正相关,并与NK细胞激活标志物CD69(r=0.51,P<0.05)和KLRB1(r=0.37,P<0.05)的表达显著正相关。双荧光素酶报告基因实验结果显示,RSPO3敲减后Wnt荧光素酶活性下调(1.0±0.0 vs 0.45±0.09,P<0.05),而RSPO3过表达后Wnt荧光素酶活性上调(1.0±0.0 vs 1.75±0.14,P<0.05)。结论:RSPO3能在体内显著抑制结直肠癌移植瘤的生长,并能增加移植瘤组织中NK细胞浸润,RSPO3是一个潜在的结直肠癌的抑制基因。
[关键词] R-脊椎蛋白3;自然杀伤细胞;结直肠癌;肿瘤生长
[Abstract] Background and purpose: The occurrence and development of colorectal cancer involve the activation of multiple oncogenes and the inactivation of tumor suppressor genes. At present, the role of wild-type R-spondin 3 (RSPO3) in the growth of colorectal cancer is still unclear. This study aimed to investigate the effect of RSPO3 on the growth of colorectal cancer and explore the underlying mechanisms. Methods: RSPO3 expression in various tumor tissues and adjacent tissues and the relationships between RSPO3 expression and natural killer (NK) cell infiltration and activating molecules in colorectal cancer tissues were analyzed by bioinformatics. RSPO3-knockdown SW480 (SW480-RSPO3-KD) and RSPO3-overexpressed HCT116 (HCT116-RSPO3-OE) gene modification cell line as well as their control cell lines were established by short hairpin RNA (shRNA) and lentiviral infection. Cell proliferation in vitro were detected using cell counting kit-8 (CCK-8). Cell cycle of colon cancer stable cells and the proportion of NK cells in spleen and transplanted tumor tissues of nude mice were determined by flow cytometry. The growth of SW480-RSPO3-KD and HCT116-RSPO3-OE stable cell subcutaneous xenografts in vivo was observed in BALB/c nude mice. The Wnt gene activity was detected by dual-luciferase reporter system. Results: Expression level of RSPO3 was low in a variety of solid tumor tissues including colorectal cancer tissues compared to their adjacent normal tissues. RSPO3 knockdown or overexpression did not affect cell proliferation (P>0.05) and cell cycle in colon cancer cells in vitro (P>0.05). Surprisingly, RSPO3 knockdown significantly promoted the growth of SW480 subcutaneous xenograft tumor (260.2±162.4 vs 1 311.7±570.1, P<0.05). RSPO3 overexpression significantly inhibited the growth of HCT116 subcutaneous xenograft tumor (1 549.0±241.2 vs 512.1±250.0, P<0.05). Flow cytometry analysis showed that the percentages of NK cells in spleen and xenograft tissues were significantly decreased in mice transplanted with RSPO3-KD cells compared with control nude mice transplanted with control cells (spleen:6.42±0.94 vs 5.25±0.59, P=0.04; transplanted tumor: 8.27±0.29 vs 6.48±1.48, P=0.04). The percentage of NK cells was significantly increased in mice transplanted with RSPO3-OE cells compared with control mice transplanted with control cells (spleen: 5.29±0.16 vs 7.02±0.49, P=0.01; transplanted tumor: 6.39±0.39 vs 8.14 ±0.34, P<0.05). The Cancer Genome Atlas (TCGA) data analysis showed that expression of RSPO3 was positively correlated with the expressions of NK cell markers CD56 (R=0.58, P<0.05) and CD16 (R=0.64, P<0.05), and with the expressions of NK cell activation markers CD69 (R=0.51, P<0.05) and KLRB1 (R=0.37, P<0.05). RSPO3 knockdown inhibited the activity of Wnt luciferase (1.00±0.00 vs 0.45±0.09, P<0.05), and RSPO3 overexpression increased the Wnt luciferase activity in colon cancer cells (1.00±0.00 vs 1.75±0.14, P<0.05). Conclusion:RSPO3 can significantly inhibit the growth of colorectal cancer xenograft in vivo and increase NK cell frequency and Wnt activity in colon cancer cells. RSPO3 may be a potential colorectal cancer suppressor.
[Key words] R-spondin 3; Natural killer cell; Colorectal cancer; Tumor growth
R-脊椎蛋白(R-spondin,RSPO)家族共有4种分泌蛋白,包括RSPO3。大量研究发现, RSPO3在发育[1]、成骨[2-3]、血管生成和血管功能调节[4]、造血[5]、体脂分布[6]、胃腺的分泌分化[7]等方面发挥重要的生理作用。此外,RSPO3还是肠道产生的最主要的RSPO家族蛋白,主要来源于肠黏膜固有层中的淋巴内皮细胞[8],用于协调肠道干细胞的自我更新和分化,维持肠隐窝的稳态[9]。多项研究表明, RSPO3主要通过激活Wnt信号通路维持肠干细胞的更新[10]和介导多种疾病[11]。
RSPO家族蛋白在肿瘤方面的作用仍存在争议。RSPO1被认为是急性和慢性淋巴细胞白血病中潜在的肿瘤抑制基因[12]。RSPO2在稳定表达叉头框Q1(forkhead box Q1,FOXQ1)基因的人结直肠癌细胞系中表达上调[13],并被认为是与裸小鼠乳腺肿瘤发生相关的致癌基因[14]。关于RSPO3在肿瘤发生、发展中的作用,既往研究几乎均认为它具有促癌功能,例如,已有研究显示,RSPO3能够促进乳腺癌[15]、卵巢癌[16]、膀胱癌[17]和绒毛膜上皮癌[18]等恶性肿瘤的生长和转移。但最近的一项研究[19]发现,肿瘤微环境来源的RSPO3能通过募集激活自然杀伤(natural killer,NK)细胞抑制胰腺癌、黑色素瘤和乳腺癌的生长。因此,RSPO家族蛋白可能在肿瘤发生、发展的不同阶段发挥不同的作用,特别是RSPO3,目前有关RSPO3在结直肠癌生长中的作用罕见报道。
结直肠癌是中国常见的恶性肿瘤之一,结直肠癌的发生、发展涉及多种癌基因的激活和抑癌基因的失活。先前研究[20]发现,约7.5%的结直肠癌患者存在K型蛋白酪氨酸磷酸酶受体K (protein tyrosine phosphatase receptor type K,PTPRK)(e7)-RSPO3(e2)基因融合突变,该突变能够激活Wnt信号转导通路并促进结直肠癌的生长,但野生型RSPO3在结直肠癌生长中的作用目前尚不清楚。本研究旨在探讨RSPO3对结直肠癌生长的影响并探索其潜在机制。
1 材料和方法
1.1 细胞、动物、组织及主要试剂
所有细胞均购自美国典型培养物保藏中心(American Type Culture Collection,ATCC)细胞库,包括正常肠上皮细胞系NCM460和结肠癌细胞系SW620、HT29、RKO、LOVO、HCT116、DLD1、SW480。6周龄BALB/c裸小鼠购自上海吉辉实验动物饲养有限公司。RPMI-1640培养基、胰蛋白酶和青霉素-链霉素混合液购自江苏凯基生物技术股份有限公司,胎牛血清购自美国Gibco公司,短发夹RNA(short hairpin RNA,shRNA)和慢病毒购自吉满生物科技(上海)有限公司,反转录和实时定量聚合酶链反应(real time-polymerase chain reaction,RT-PCR)试剂购自翌圣生物科技(上海)股份有限公司,RSPO3抗体购自美国Sigma-Aldrich公司,引物购自生工生物工程(上海)股份有限公司,酶联免疫吸附试验(enzyme-linked immunosorbent assay,ELISA)试剂盒购自美国R&D Systems公司,流式抗体购自美国BD Bioscience公司,双荧光素酶试剂盒E1910购自美国Promega公司。
1.2 PCR
采用TRIzol 提取RNA后,取1 μg反转录为cDNA,设计RSPO3的上游引物序列为5‘-AGGCGCCAGCGAAGAAT-3‘,下游引物序列为5‘-TCCAGACTTTTGCTGTCAGGT-3‘。反转录按照Hifair® Ⅲ One Step RT-PCR SYBR Green Kit说明书操作。取2 μL cDNA进行PCR,通过琼脂糖凝胶电泳观察表达水平。
1.3 构建基因修饰细胞株
使用重组质粒(RSPO3-psin-EF2-puro、RSPO3 shRNA1、RSPO3 shRNA2)、空载对照质粒(psin-EF2-puro、pSU-PER-retro-puro)、反转录包装质粒、慢病毒包装质粒和HEK-293T细胞包装病毒,病毒感染细胞后,用含1 μg/mL嘌呤霉素的培养基筛选阳性细胞并扩大培养,分别建立RSPO3过表达、干扰的稳定细胞株,提取RNA后采用反转录PCR验证基因修饰细胞株的构建是否成功。
1.4 ELISA
用包被缓冲液稀释特异性抗体球蛋白后加入凹孔,4 ℃过夜。用含0.05%吐温-20的洗涤缓冲液洗3次后加入含抗原的细胞培养上清液标本, 37 ℃温育2 h。移去包被液后洗涤,加入0.2 mL稀释缓冲液稀释的酶标记特异性抗体溶液,37 ℃作用2 h后再次洗涤并加入底物溶液,室温温育30 min后终止,用酶标仪测定492 nm处的吸光度(D)值。
1.5 细胞计数试剂盒-8(cell counting kit-8,CCK-8)检测
待测细胞以4 000个细胞/100 μL/孔的密度接种在96孔培养板中,根据说明书,使用CCK-8检测细胞活力。
1.6 流式细胞术分析
裸小鼠脾脏经70 μm滤网研磨或皮下瘤组织经Ⅳ型胶原酶处理为单细胞悬液后,使用抗裸小鼠CD16/32封闭Fc受体,随后用PE标记的CD49b、AF700标记的CD3和4‘,6-二脒基-2-苯基吲哚(4‘,6-diamidino-2-phenylindole,DAPI)流式抗体4 ℃避光染色30 min,上机并圈门分析NK细胞(CD3-CD49b+)[18]。细胞周期采用AmcYan染色,细胞经70%乙醇溶液固定后于-20 ℃过夜,恢复至室温后染色30 min,并采用流式细胞术和Flowjo软件分析。
1.7 皮下移植瘤实验
每只裸小鼠右侧背部接种5×106个肿瘤细胞,待成瘤后每2 d测量长径(L)和短径(W),肿瘤体积 = L×W2/2[21]。
1.8 双荧光素酶报告基因实验
构建Wnt基因的3‘非翻译区(3‘-untranslated region,3‘-UTR)-荧光素酶报告质粒,转染后按照Dual-Luciferase® Reporter Assay System试剂盒说明书操作,使用荧光素酶报告基因检测仪进行测定,以荧光素酶活性和Renilla活性的比值计算实验结果[22]。
1.9 生物信息学分析
通过Oncomine数据库(https://www. oncomine.org/resource/login.html)在线分析泛癌组织的基因芯片数据中RSPO3的表达[23],通过癌细胞系百科全书(Cancer Cell Line Encyclopedia,CCLE)数据库(https://sites. broadinstitute.org/ccle)在线分析泛癌细胞系测序数据中RSPO3的表达[24],通过基于癌症基因组图谱(The Cancer Genome Atlas,TCGA)转录组测序数据的肿瘤免疫系统交互数据库(Tumor-Immune System Interactions Database,TISIDB)(http://cis.hku.hk/TISIDB/)在线分析RSPO3与免疫浸润淋巴细胞的相关性[25],通过基因表达谱交互分析(Gene Expression Profiling Interactive Analysis,GEPIA)数据库(http://gepia.cancer-pku.cn/detail.php)基于TCGA结肠癌和直肠癌数据库中的转录组测序数据分析RSPO3表达与NK细胞相关分子标志物表达的相关性[26]。
1.10 统计学处理
基于GraphPad Prism 7.0和SPSS 17.0软件对数据进行分析,根据资料分布情况,两组比较采用t检验或非参数检验,P<0.05为差异有统计学意义。
2 结 果
2.1 RSPO3在结直肠癌及泛癌组织中的表达
通过Oncomine数据库挖掘发现,RSPO3在多种实体肿瘤组织中的表达显著低于癌旁组织,包括结直肠癌[结肠癌:1.17(0.42~2.01) vs 2.51(2.30~3.73),P<0.000 1;直肠癌:1.11(0.39~1.35) vs 3.06(2.62~3.78),P <0.000 1]、乳腺癌[1.50(1.02~2.19) vs 4.08(3.50~4.52),P<0.000 1]、胶质瘤[0.25(0.11~0.51) vs 2.90(2.80~3.20),P<0.000 1]、头颈鳞癌[0.58(0.25~1.14) vs 1.15(1.10~1.89),P<0.000 1]及前列腺癌[1.97(1.18~2.75) vs 3.70(2.24~3.72),P<0.000 1]等(图1A、1B)。CCLE数据库中大多数肿瘤细胞系的RSPO3表达水平较低,包括结直肠癌细胞系(图1C)。TISIDB数据库分析发现,RSPO3表达与多种免疫细胞的浸润呈正相关,其中,结直肠癌中的RSPO3表达水平与NK细胞的浸润呈显著正相关(结肠癌:r=0.649,P<0.05;直肠癌:r=0.629,P<0.05),详见图1D。
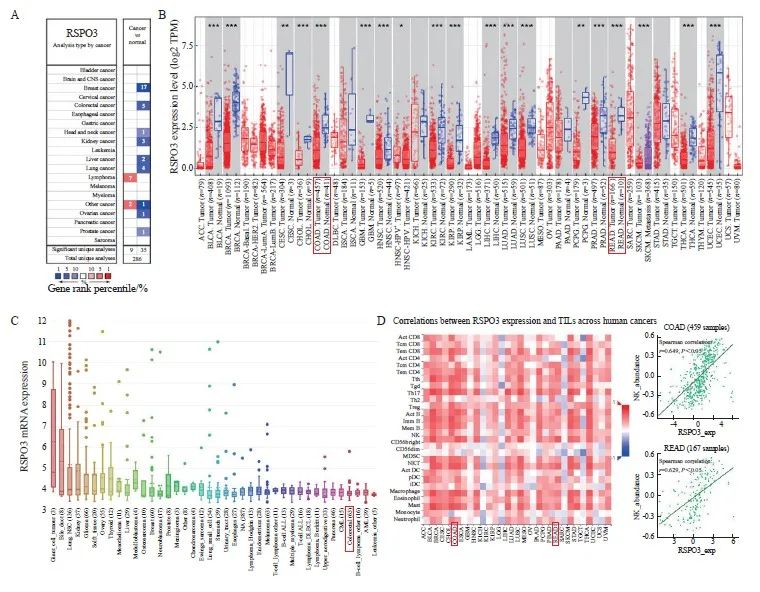
图1 RSPO3在泛癌种中的表达情况
Fig. 1 Expression of RSPO3 in pancarcinoma
A, B: The Oncomine database suggests that RSPO3 is relatively low expressed in a variety of human solid tumors, including colorectal cancer; C: Expression of RSPO3 in various tumor cell lines in CCLE database; D: TISIDB database showed the correlation between RSPO3 expression and various immunoinfiltrated lymphocytes, and RSPO3 expression in colorectal cancer was significantly positively correlated with NK cells. TIL: Tumor infiltrating lymphocytes. *: P<0.05; **: P<0.01; ***: P<0.001.
2.2 构建RSPO3敲减或过表达的肠癌细胞和对照细胞株
通过ELISA检测人结肠上皮细胞系NCM460和多种肠癌细胞系上清液中RSPO3的表达, SW620、HT29、RKO、LOVO、HCT116及DLD1的RSPO3表达水平均比NCM460低,而SW480的RSPO3表达水平比NCM460高(图2A)。因此,本研究进一步利用shRNA,构建并验证了RSPO3敲减的SW480-RSPO3-KD细胞株和对照细胞株SW480-Ctrl,此外,本研究还构建并验证了RSPO3过表达的HCT116-RSPO3-OE细胞株和对照细胞株HCT116-Ctrl,结果见图2B、2C。
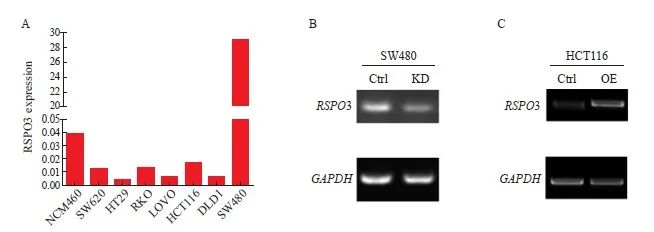
图2 构建RSPO3敲减或过表达的肠癌细胞株
Fig. 2 Construction of RSPO3 knockdown or overexpressed colon cancer cell lines
A: RSPO3 expression in human colon epithelial cells NCM460 and a variety of colon cancer cell lines; B: shRNA knockdown RSPO3 expression in SW480 cell line; C: Lentivirus infection induced RSPO3 overexpression in HCT116 cell line. KD: Knock down; OE: Overexpression.
2.3 RSPO3敲减或过表达在体内外对SW480和HCT116细胞生长的影响
通过CCK-8实验检测体外细胞增殖,通过AmcYan染色和流式细胞术分析检测细胞周期,结果显示,RSPO3在体外不影响SW480和HCT116细胞增殖(SW480:2.98±0.01 vs 2.85±0.02 vs 3.01±0.00,P>0.05;HCT116:2.01±0.01 vs 2.02±0.01,P>0.05),对结肠癌细胞SW480(G1期:25.02±1.58 vs 18.98±1.33;S期:52.26±4.01 vs 55.05±3.02;G2期/M期:20.03±3.03 vs 21.85±2.02)和HCT116(G1期:56.95±0.78 vs 59.25±3.97;S期:14.97±1.52 vs 16.27±1.05;G2期/M期:28.08±0.78 vs 24.47±4.48)的细胞周期也无显著影响(P>0.05,图3A~3D)。另外,本研究观察到,RSPO3敲减在裸小鼠体内能显著促进SW480移植瘤的生长(260.2±162.4 vs 1 311.7±570.1,P<0.05,图3E~3G),而RSPO3过表达在裸小鼠体内则显著抑制HCT116移植瘤的生长(1 549.0±241.2 vs 512.1±250.0,P <0.05,图3H~3J)。上述结果提示RSPO3表达改变在体内能够影响结肠癌的生长。
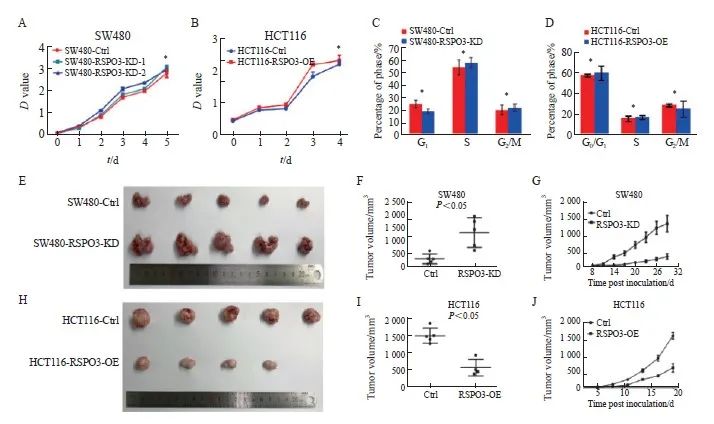
图3 RSPO3在体内外对 SW480或HCT116细胞生长的影响
Fig. 3 Effects of RSPO3 on the growth of SW480 or HCT116 cell line in vitro and in vivo
A, B: The effects of RSPO3 knockdown or overexpression on cell proliferation of SW480 or HCT116 cell line were analyzed by CCK-8; C, D: Flow cytometry was used to detect the effects of RSPO3 knockdown or overexpression on SW480 or HCT116 cell cycles; E, F, G: Effects of RSPO3 knockdown on the growth of SW480 subcutaneous xenograft tumors; H, I, J: Effects of RSPO3 overexpression on the growth of HCT116 subcutaneous xenograft tumors.*: P>0.05.
2.4 RSPO3敲减或过表达对NK细胞比例的影响
到达观察终点后,处死裸小鼠并取脾脏和移植瘤组织以制备单细胞悬液,利用流式细胞术检测CD3-CD49+ NK细胞的比例,发现RSPO3敲减后裸小鼠的脾脏和移植瘤组织中的NK细胞比例显著减少(脾脏:6.42±0.94 vs 5.25±0.59,P=0.04;移植瘤:8.27±0.29 vs 6.48±1.48,P=0.04),而RSPO3过表达后裸小鼠的脾脏和移植瘤组织中的NK细胞比例显著增多(脾脏:5.29±0.16 vs 7.02±0.49,P=0.01;移植瘤:6.39±0.39 vs 8.14±0.34,P<0.05,图4)。上述结果提示RSPO3表达与NK细胞浸润增加有关。
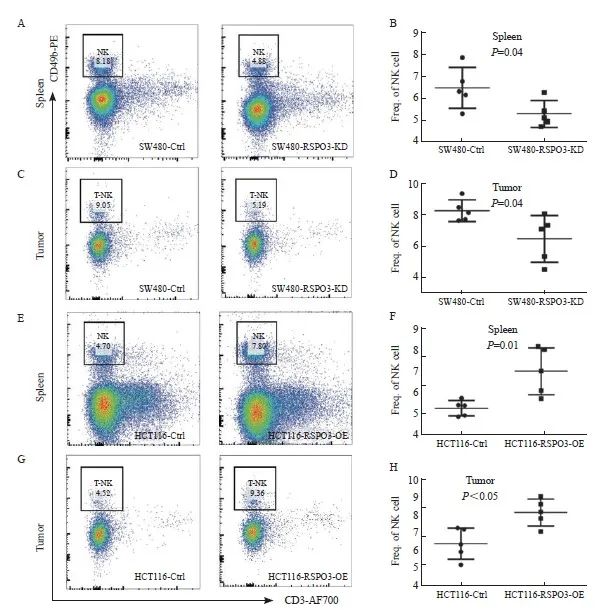
图4 RSPO3在体内影响NK细胞的比例
Fig. 4 RSPO3 affects the proportion of NK cells in vivo
A, B: Flow cytometry was used to analyze the proportion of NK cells in single cell suspension of spleen after transplantation of SW480-RSPO3-KD or SW480-Ctrl subcutaneous xenograft tumor; C, D: Flow cytometry was used to analyze the proportion of NK cells in the subcutaneous tumor of SW480-RSPO3-KD or SW480-Ctrl; E, F: Flow cytometry was used to analyze the proportion of NK cells in spleen single-cell suspension after transplantation of HCT116-RSPO3-OE or HCT116-Ctrl subcutaneous xenograft tumor; G, H: Flow cytometry was used to analyze the proportion of NK cells in the subcutaneous xenograft tumor of HCT116-RSPO3-OE or HCT116-Ctrl.
2.5 RSPO3与NK细胞及其激活标志物的表达呈显著正相关
基于TCGA数据库中结肠癌和直肠癌转录组测序数据的相关性分析结果表明,RSPO3表达水平与NK细胞的表面标志物分子CD56(基因名NCAM1,r=0.58,P<0.05)和CD16(基因名FCGR3A,r= 0.64,P<0.05)的表达显著正相关,此外,RSPO3表达水平还与NK激活的标志物分子CD69(r= 0.51,P<0.05)和KLRB1(r=0.37,P<0.05)的表达显著正相关(图5),表明RSPO3可能不仅能上调NK细胞,还可能参与NK细胞的激活。
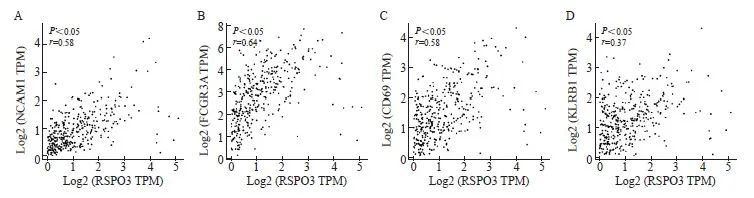
图5 RSPO3表达水平与NK细胞标志物及其激活分子标志物的相关性
Fig. 5 Correlation analysis of RSPO3 expression level with NK cell markers and NK activated markers
A: Spearman correlation analysis based on TCGA colorectal cancer database showed that RSPO3 was highly positively correlated with CD56 (NCAM1), r= 0.58, P<0.05; B: RSPO3 was highly positively correlated with CD16 (FCGR3A), r= 0.64, P<0.05; C: RSPO3 was highly positively correlated with CD69, r= 0.51, P<0.05; D: RSPO3 was moderately positively correlated with KLRB1 (r= 0.37, P<0.05).
2.6 RSPO3敲减或过表达对Wnt信号转导通路的影响
双荧光素酶报告基因实验结果提示,与对照组相比,RSPO3敲减后Wnt荧光素酶活性下调(1.0±0.0 vs 0.45±0.09,P<0.05),而RSPO3过表达后Wnt荧光素酶活性上调(1.0±0.0 vs 1.75±0.14,P<0.05,图6)。
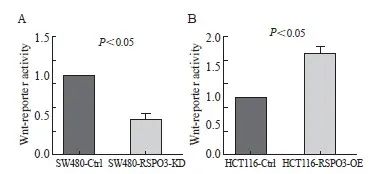
图6 RSPO3敲减或过表达对Wnt荧光素酶活性的影响
Fig. 6 Effect of RSPO3 knockdown or overexpression on luciferase activity of Wnt
A: Wnt luciferase activity was down-regulated after RSPO3 knockout; B: Wnt luciferase activity was up-regulated after RSPO3 overexpression.
3 讨 论
既往研究通常认为RSPO3在乳腺癌[15]、卵巢癌[16]、膀胱癌[17]和绒毛膜上皮癌[18]等恶性肿瘤中发挥促癌作用。先前研究[20]显示,在7.5%的结肠癌组织中存在PTPRK(e7)-RSPO3(e2)融合突变,RSPO3融合突变可促进结肠癌的生长。本研究发现,RSPO3在结直肠癌组织和多数结直肠癌细胞系中低表达。通过构建稳定过表达RSPO3的HCT116细胞株和RSPO3基因敲减的SW480细胞株,发现RSPO3过表达显著抑制了结肠癌HCT116细胞的皮下移植瘤的生长,而RSPO3敲减则显著促进了SW480细胞的移植瘤生长,证实RSPO3能够抑制结直肠癌在体内的生长,是一个潜在的结肠癌的抑制基因。
目前对RSPO3抑制结直肠癌生长的机制尚缺乏研究。本研究发现,RSPO3过表达或基因沉默在体外并不影响SW480和HCT116细胞增殖和细胞周期,而仅在体内移植瘤实验中观察到RSPO3具有显著的抑瘤作用,提示RSPO3可能是通过调节体内的肿瘤免疫微环境发挥抑瘤作用。T细胞和NK细胞是体内发挥抗肿瘤作用的主要免疫杀伤细胞。本研究采用T细胞免疫缺陷的裸小鼠移植瘤模型,观察到RSPO3过表达显著抑制了结直肠癌移植瘤的生长,而RSPO3敲减则促进了结直肠癌移植瘤的生长,提示RSPO3抑制肿瘤的作用不依赖于T细胞的免疫功能,NK细胞可能发挥重要作用。
NK细胞是典型的固有免疫细胞,具有非主要组织相容性复合体限制性,其数量和比例的增加有利于直接杀伤肿瘤细胞[27]。本研究采用流式细胞术分析证实,接种RSPO3敲减的SW480稳定转染细胞后,裸小鼠的脾脏和肿瘤组织中的NK细胞比例均显著减少,而接种RSPO3过表达的HCT116细胞后,裸小鼠的脾脏和肿瘤组织中的NK细胞数量均显著增多,提示RSPO3能够促进外周循环和肿瘤微环境中的NK细胞浸润,这一结果与Tang等[19]在胰腺癌和黑色素瘤中的发现一致。Tang等[19]研究发现,RSPO3过表达能够抑制裸小鼠体内黑色素瘤和胰腺癌移植瘤的生长,在接种RSPO3过表达的肿瘤细胞的移植瘤中,NK细胞的比例显著升高,并且RSPO3促进黑色素瘤和胰腺癌肿瘤组织中NK细胞的颗粒酶和穿孔素的分泌,以及增加CD69的表达,而清除NK细胞降低了RSPO3抑制黑色素瘤和胰腺癌移植瘤生长的作用,提示NK细胞介导了RSPO3抑制肿瘤生长的作用。本研究的生物信息学分析结果也证实,RSPO3表达水平与结直肠癌中NK细胞的浸润水平,与NK细胞表面标志物CD16及CD56的表达水平呈显著正相关,与NK细胞激活标志物CD69及KLRB1的表达水平也呈显著正相关,表明RSPO3过表达不仅会增加NK细胞的浸润,而且可能增加NK细胞的活性。本研究结果初步证实NK细胞可能在介导RSPO3抑制结直肠癌生长中发挥一定的作用。NK细胞是否为介导RSPO3抑制结直肠癌生长的关键免疫细胞,仍有待将来通过NK细胞清除实验来进一步验证。
有研究[28]表明,RSPO3参与调控经典的Wnt/β-catenin信号转导通路,此外,RSPO3通过结合多配体聚糖4受体诱导非经典的Wnt/平面细胞极化(planner cell polarity,PCP)信号转导通路。本研究发现RSPO3敲减后SW480细胞的Wnt基因活性显著下调,过表达RSPO3后HCT116细胞的Wnt基因活性显著上调,然而RSPO3在体内的抑瘤作用是否与Wnt信号转导通路直接相关还需更多的实验来验证。
综上,本研究发现RSPO3能够在体内显著抑制结直肠癌的生长,并参与上调NK细胞和Wnt信号转导通路。将来需要进一步证实RSPO3是否主要通过调控NK细胞或Wnt信号转导通路抑制结直肠癌的生长,并探索通过恢复结直肠癌中的RSPO3表达水平来治疗结直肠癌的可行性,从而为结直肠癌的治疗提供一个新靶标。
利益冲突声明:所有作者均声明不存在利益冲突。
[参考文献]
[1]AOKI M, MIEDA M, IKEDA T, et al. R-spondin3 is required for mouse placental development[J]. Dev Biol, 2007, 301(1): 218-226.
[2]KNIGHT M N, HANKENSON K D. R-spondins: novel matricellular regulators of the skeleton[J]. Matrix Biol, 2014, 37: 157-161.
[3]NILSSON K H, HENNING P, EL SHAHAWY M, et al. RSPO3 is important for trabecular bone and fracture risk in mice and humans[J]. Nat Commun, 2021, 12(1): 4923.
[4]KAZANSKAYA O, OHKAWARA B, HEROULT M, et al. The Wnt signaling regulator R-spondin 3 promotes angioblast and vascular development[J]. Development, 2008, 135(22): 3655-3664.
[5]KURTOVA A V, HEINLEIN M, HAAS S, et al. Disruption of stem cell niche-confined R-spondin 3 expression leads to impaired hematopoiesis[J]. Blood Adv, 2023, 7(4): 491-507.
[6]LOH N Y, MINCHIN J E N, PINNICK K E, et al. RSPO3 impacts body fat distribution and regulates adipose cell biology in vitro[J]. Nat Commun, 2020, 11(1): 2797.
[7]KUROKAWA K, WANG T C, HAYAKAWA Y. R-spondin 3 governs secretory differentiation in the gastric oxyntic glands[J]. J Clin Invest, 2022, 132(21): e163380.
[8]OGASAWARA R, HASHIMOTO D, KIMURA S, et al. Intestinal lymphatic endothelial cells produce R-spondin 3[J]. Sci Rep, 2018, 8(1): 10719.
[9]GREICIUS G, KABIRI Z, SIGMUNDSSON K, et al. PDGFRα+ pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo[J]. Proc Natl Acad Sci U S A, 2018, 115(14): E3173-E3181.
[10]YAN K S, JANDA C Y, CHANG J L, et al. Non-equivalence of Wnt and R-spondin ligands during Lgr5+ intestinal stem-cell self-renewal[J]. Nature, 2017, 545(7653): 238-242.
[11]ANNUNZIATO S, SUN T, TCHORZ J S. The RSPO-LGR4/5-ZNRF3/RNF43 module in liver homeostasis, regeneration, and disease[J]. Hepatology, 2022, 76(3): 888-899.
[12]KUANG S Q, TONG W G, YANG H, et al. Genome-wide identification of aberrantly methylated promoter associated CpG islands in acute lymphocytic leukemia[J]. Leukemia, 2008, 22(8): 1529-1538.
[13]KANEDA H, ARAO T, TANAKA K, et al. FOXQ1 is overexpressed in colorectal cancer and enhances tumorigenicity and tumor growth[J]. Cancer Res, 2010, 70(5): 2053-2063.
[14]THEODOROU V, KIMM M A, BOER M, et al. MMTV insertional mutagenesis identifies genes, gene families and pathways involved in mammary cancer[J]. Nat Genet, 2007, 39(6): 759-769.
[15]TER STEEGE E J, BOER M, TIMMER N C, et al. R-spondin 3 is an oncogenic driver of poorly differentiated invasive breast cancer[J]. J Pathol, 2022, 258(3): 289-299.
[16]GU H F, TU H, LIU L L, et al. RSPO3 is a marker candidate for predicting tumor aggressiveness in ovarian cancer[J]. Ann Transl Med, 2020, 8(21): 1351.
[17]CHEN Z H, ZHOU L J, CHEN L, et al. RSPO3 promotes the aggressiveness of bladder cancer via Wnt/β-catenin and Hedgehog signaling pathways[J]. Carcinogenesis, 2019, 40(2): 360-369.
[18]CHEN Z L, ZHANG J Z, YUAN A W, et al. R-spondin 3 promotes the tumor growth of choriocarcinoma JEG-3 cells[J]. Am J Physiol Cell Physiol, 2020, 318(3): C664-C674.
[19]TANG Y T, XU Q, HU L, et al. Tumor microenvironment-derived R-spondins enhance antitumor immunity to suppress tumor growth and sensitize for immune checkpoint blockade therapy[J]. Cancer Discov, 2021, 11(12): 3142-3157.
[20]SESHAGIRI S, STAWISKI E W, DURINCK S, et al. Recurrent R-spondin fusions in colon cancer[J]. Nature, 2012, 488(7413): 660-664.
[21]TANG Y, ZHOU C, LI Q L, et al. Targeting depletion of myeloid-derived suppressor cells potentiates PD-L1 blockade efficacy in gastric and colon cancers[J]. Oncoimmunology, 2022, 11(1): 2131084.
[22]焦红丽, 王珺娆, 胡敏萱, 等. SAFB通过调节Wnt信号通路活性促进结直肠癌增殖[J]. 临床与实验病理学杂志, 2022, 38(4): 385-391
JIAO H L, WANG J R, HU M X, et al. SAFB promotes colorectal cancer proliferation by regulating Wnt signaling pathway activity[J]. Chin J Clin Exp Pathol, 2022, 38(4): 385-391
[23]RHODES D R, KALYANA-SUNDARAM S, MAHAVISNO V, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18 000 cancer gene expression profiles[J]. Neoplasia, 2007, 9(2): 166-180.
[24]GHANDI M, HUANG F W, JANÉ-VALBUENA J, et al. Next-generation characterization of the cancer cell line encyclopedia[J]. Nature, 2019, 569(7757): 503-508.
[25]RU B B, WONG C N, TONG Y, et al. TISIDB: an integrated repository portal for tumor-immune system interactions[J]. Bioinformatics, 2019, 35(20): 4200-4202.
[26]TANG Z F, LI C W, KANG B X, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses[J]. Nucleic Acids Res, 2017, 45(W1): W98-W102.
[27]RUSSICK J, TORSET C, HEMERY E, et al. NK cells in the tumor microenvironment: prognostic and theranostic impact. Recent advances and trends[J]. Semin Immunol, 2020, 48: 101407.
[28]OHKAWARA B, GLINKA A, NIEHRS C. RSPO3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis[J]. Dev Cell, 2011, 20(3): 303-314.


