【协和医学杂志】放射性药物联合免疫检查点抑制剂协同抗肿瘤研究新进展
时间:2023-08-11 21:42:23 热度:37.1℃ 作者:网络
近10年来,免疫检查点抑制剂(ICI)在肿瘤治疗领域取得了重大突破。2011年,第一个靶向细胞毒性T淋巴细胞抗原4(CTLA-4)的ICI伊匹单抗(Ipilimumab)获得美国食品药品监督管理局(FDA)批准用于晚期黑色素瘤的治疗,此后多个靶向程序性死亡受体1及其配体(PD-1/PD-L1)及淋巴细胞激活基因-3(LAG-3)的ICI陆续上市[1-2]。
虽然ICI在多种转移性、难治性恶性肿瘤中获得了持久反应性,但患者对于ICI单药的响应率仅为20%~40%[3-4]。而ICI治疗产生的免疫相关不良事件及获得性耐药均可导致疾病进展[4],因此,与其他治疗方式联合成为ICI治疗的新方向[2]。
研究表明,外照射放疗(EBRT)可引起肿瘤细胞DNA损伤、信号转导调节以及肿瘤免疫微环境(TIME)重塑[5]。EBRT通过均匀的低传能线密度(LET)射线,包括X射线及γ射线(0.2 keV/μm),以高吸收剂量率(1~2 Gy/min)靶向照射部位[6],导致肿瘤细胞DNA损伤,激活cGAS-STING-IRF3-Type Ⅰ干扰素(IFN)信号级联反应,招募调节性T细胞(Tregs)、髓源性抑制细胞(MDSC)等免疫抑制细胞,诱导适应性免疫反应[7-8],同时引起TIME中PD-L1表达上调,负性调节肿瘤浸润T细胞引起肿瘤耐药或复发[5,9]。
因此,多个研究证明了EBRT与ICI的协同作用[10-11]。Twyman-Saint等[12]发现,PD-L1抗体的加入可逆转EBRT联合CTLA-4抗体引起的T细胞耗竭,增加黑色素瘤对联合治疗的响应率。然而EBRT作为一种局部治疗方式,即便通过免疫介导引起“远隔效应”,也仅有少数患者能因此获益[13-14]。
靶向放射性核素治疗(TRT)是一种内放射治疗,其中放射性药物通过全身给药特异性定位于靶点,向肿瘤传递细胞毒性辐射。TRT以低吸收剂量率(<1 Gy/h)长时间照射靶部位,射线的类型与放射性核素有关,包括α、β粒子及俄歇电子。
LET及最大穿透深度也与核素衰变类型相关,α粒子具有较高的LET(50~230 keV/μm)和较小的穿透深度(50~100 μm);β粒子则具有更低的LET(0.2 keV/μm)和几十至数百个细胞(mm级别)的穿透距离;俄歇电子的LET为4~25 keV/μm,组织穿透最大深度<1 μm[6]。
因此,EBRT对于TIME的调节作用不能简单地外推至TRT[15-16]。研究发现,高剂量TRT诱导DNA双链断裂,可上调膜联蛋白A1及钙网蛋白[17],激活Ⅰ型IFN,其幅度和时间过程与EBRT相当,而低剂量的TRT则表现出明显的免疫调节作用,包括促炎细胞因子及细胞毒性T细胞(CTLs)的浸润[18-19]。
然而,EBRT通过同源重组、非同源末端连接及碱基切除修复诱导DNA双链修复,TRT却通过跨损伤合成修复受损的DNA[20]。同时,Grzmil等[20]通过蛋白质组学研究发现,EBRT与TRT诱导了不同信号通路的激活,TRT主要影响表皮生长因子受体、丝裂原活化蛋白激酶、整合素和雌激素受体的信号转导。
本文将聚焦于该领域最新研究进展,对TRT联合PD-L1抗体治疗的模式及其机制进行综述,以期指导临床实践。
1 靶向治疗放射性药物联合ICI治疗
1.1 β核素放射性药物联合ICI治疗
1.1.1 碘[131I]
131I 衰变发射β粒子及γ光子,是最早用于TRT的放射性核素。Rouanet等[17]比较了131I-ICF01012与ICI的不同组合方案,发现无论是否加入PD-1/PD-L1抗体,对于治疗效果均无显著影响,但观察到了TRT联合CTLA-4抗体后T细胞衰竭相关基因CD274、LAG3和Eomes增加。而131I-MnO2-BSA作为一种放射增敏剂,可改善肿瘤乏氧,诱导肿瘤细胞免疫原性死亡,上调PD-L1的表达,抑制原发及转移瘤的生长[21]。
1.1.2 钇[90Y]
90Y具有纯β发射、能量强、半衰期短的特点,在Patel等[19]的研究中,发现中等剂量的EBRT与低剂量90Y-NM600(1.85 MBq)对于提高ICI疗效的作用是互补的。具体来说,联合治疗增加了总免疫细胞、CTLs、效应记忆T细胞(TEM)和γδ T细胞的数量,进而增加IFN-γ分泌,起到缓解肿瘤负担及减少自发转移的作用,联合中等剂量EBRT诱导远隔效应,可进一步缓解原发及转移瘤负担[19]。
另有研究将此策略应用于Lewis肺癌模型治疗,仅用EBRT联合CTLA-4抗体治疗能够减少肿瘤转移,但在联合了90Y-NM600治疗后,能达到肿瘤完全缓解并诱导免疫记忆[22]。但Potluri等[23]研究发现,90Y-NM600联合PD-1抗体治疗对前列腺癌无效,其原因是PD-1抗体激活了Tregs,从而可导致免疫抑制。
1.1.3 镥[177Lu]
177Lu是最常用的TRT核素,其标记的探针177Lu-DOTATATE[24]和177Lu-PSMA-617[25]已获得美国FDA批准分别用于神经内分泌瘤及转移性去势抵抗性前列腺癌(mCRPC)。其中177Lu-DOTATATE联合纳武单抗(Nivolumab)的Ⅰ期临床试验证明了其安全性,在晚期神经内分泌肿瘤及广泛期小细胞肺癌中显示出抗肿瘤效果,总缓解率为14.3%(NCT03325816)[26]。
177Lu-DOTATATE与伊匹单抗、纳武单抗联合使用在转移性Merkel细胞癌[27]、侵袭性垂体瘤患者[28]中能够安全且有效地缓解肿瘤进展。目前仍有几项177Lu-DOTATATE联合PD-1/PD-L1抗体治疗多种恶性肿瘤的临床试验(NCT04261855,NCT03457948,NCT05583708,NCT05142696,NCT04525638)正在进行中。
Prasad 等[29]在帕博利珠单抗(Pembrolizumab)或奥拉帕尼(olaparib)治疗无效的前列腺癌患者中,联合使用177Lu-PSMA-617治疗后前列腺特异性抗原水平趋于稳定。目前联合177Lu-PSMA-617及ICI用于mCRPC患者治疗的多项临床试验(NCT03805594,NCT03658447,NCT05150236)正在开展中。
Chen等[30]用177Lu-EB-RGD联合多剂量PD-L1抗体在鼠结直肠癌模型中诱导CD8+T细胞浸润,显著抑制了肿瘤生长,且提出TRT与ICI的给药时间窗对于治疗效果至关重要的观点。在此基础上,本研究团队进一步探索了177Lu-DOTA-EB-cRGDfK(177Lu-DER)联合治疗的给药策略,在体内外证明了放射性核素刺激后PD-L1表达呈时间及剂量依赖性上调,在TRT后4 h通过尾静脉注射PD-L1抗体能够大大提高联合治疗的疗效,联合治疗组小鼠血清促炎细胞因子、CTLs及CD4+辅助T细胞(helper T cells, Th1)浸润增加,肿瘤完全缓解并获得特异性免疫记忆效应[31]。随后用64Cu-DOTA-EB-cRGDfK(64Cu-DER)验证了该给药策略的有效性,提示该策略可推广至不同的放射性核素标记的探针中[32]。
此外,177Lu-DOTA-Folate[33]、177Lu-DOTA-Y003[34]、177Lu-h8C3[35]、177Lu-LLP2A[36]及177Lu-DNP-DOTA-BSA[37]联合ICI治疗均可抑制肿瘤生长并延长荷瘤小鼠生存期。
1.2 α核素放射性药物联合ICI治疗
1.2.1 锕[225Ac]
由于α射线独特的优势,包括能量高、射程短、能够诱导DNA双链不可修复的断裂等[38],α-TRT成为新的研究热点。与β-TRT相比,α-TRT剂量极低,225Ac-PSMA-617(30 kBq)联合PD-1抗体在RM1-PGLS模型中能够抑制肿瘤生长,达到部分完全缓解[39]。然而225Ac-DOTA-anti-PD-L1 Ab (14.8 kBq)联合PD-L1抗体(322 μg)同时给药却降低了小鼠的生存率[40]。
1.2.2 砹[211At]
多聚ADP核糖聚合酶靶向的探针211At-MM4导致的DNA损伤,激活了先天免疫反应,CD4+、CD8+ T细胞及巨噬细胞浸润增加,联合PD-1抗体治疗U87MG脑胶质瘤模型达到了完全缓解,65 d无进展[41]。
Zhang等[42]开发的基于锰基纳米的放射免疫治疗促进剂211At-ATE-MnO2-BSA,联合了α-TRT、化学动力治疗及PD-L1抗体治疗。研究发现,211At-ATE-MnO2-BSA激活了树突状细胞(DCs),与PD-L1抗体联合使用可进一步增加CTLs、促炎细胞因子及 TEM浸润,有效抑制了肿瘤的生长、转移及复发[42]。
1.2.3 镭[223Ra]
二氯化镭(Xofigo,223RaCl2)经美国FDA批准已用于治疗mCRPC的骨转移,223Ra处理后促进了CTLs的杀伤作用,增加了主要组织相容性复合体(MHC)-Ⅰ和钙网蛋白的表达[43]。
在临床研究中发现,经223Ra治疗的患者免疫抑制性T细胞(表达TIM-3、PD-L1和PD-1)比例增加,同时Tregs及MDSC浸润增加,联合PD-L1抗体能够改善治疗效果[44-45]。
在一项Ⅰb期临床研究(NCT02814669)中,评估了223Ra联合阿替利珠单抗(Atezolizumab)在mCRPC患者中的安全性和有效性,但联合治疗并未表现出更好的疗效,所有治疗组的中位总生存期为16.3个月,中位无进展生存期为3个月,但却增加了治疗相关毒性[46]。同时研究也发现产生治疗相关不良反应主要与阿替利珠单抗有关,因此目前正在进行的其他临床研究将Xofigo与帕博利珠单抗(NCT03996473)、纳武单抗(NCT04109729)或阿维单抗(NCT04071236)联合使用,用于晚期前列腺癌及非小细胞肺癌的骨转移治疗。
1.2.4 其他α核素
联合治疗的给药策略也是影响疗效的重要因素,剂量分割是提高EBRT联合ICI的常见方案,而对于α-TRT,一次给药效果优于多次给药。212Pb-VMT01联合CTLA-4及PD-1抗体治疗后,43%的肿瘤完全缓解,而接受多次给药方案的肿瘤最终发生进展[47]。
Nosanchuk等[48]则发现,基于213Bi的α-TRT与联合CTLA-4抗体的治疗效果无差异。213Bi的免疫调节作用依赖于损伤相关的分子模式(DAMPs)的释放并激活DCs,治疗后肿瘤组织Tregs下调,白细胞介素(IL) -2、趋化因子C-C-基元配体 (CCL) -5及IFN-γ短暂上调,产生肿瘤杀伤作用[49-50]。
相似的机制在227Th中也得到了验证,MSLN-TTC处理后IL-6、CCL20、CXCL10和STING相关基因在体外上调,DAMPs上调导致DCs活化[51-52]。MSLN-TTC治疗后检测到CD103+ DCs迁移及CTLs的浸润,联合PD-L1抗体后进一步增强这种效应,并观察到IFN-γ、CCL3、CCL4、IL-2、IL-5和IL-10的上调,部分肿瘤完全缓解[51]。
2 靶向诊断放射性药物联合ICI治疗
据文献报道,高剂量的经典诊断探针2-[18F]FDG (74-148 MBq)治疗可抑制肿瘤生长并适度延长生存期,提示诊断核素在肿瘤治疗中具有较大潜力[53-55]。
如图1所示,本研究团队创新性地采用2-[18F]FDG联合PD-L1抗体治疗荷瘤小鼠,以静脉注射37 MBq或18.5 MBq 2-[18F]FDG后4 h联合400 μg PD-L1抗体治疗为一个疗程,两个疗程后(d0及d4)部分肿瘤完全缓解(5/8或4/8),且治愈后再次接种肿瘤细胞未发现肿瘤生长[56]。
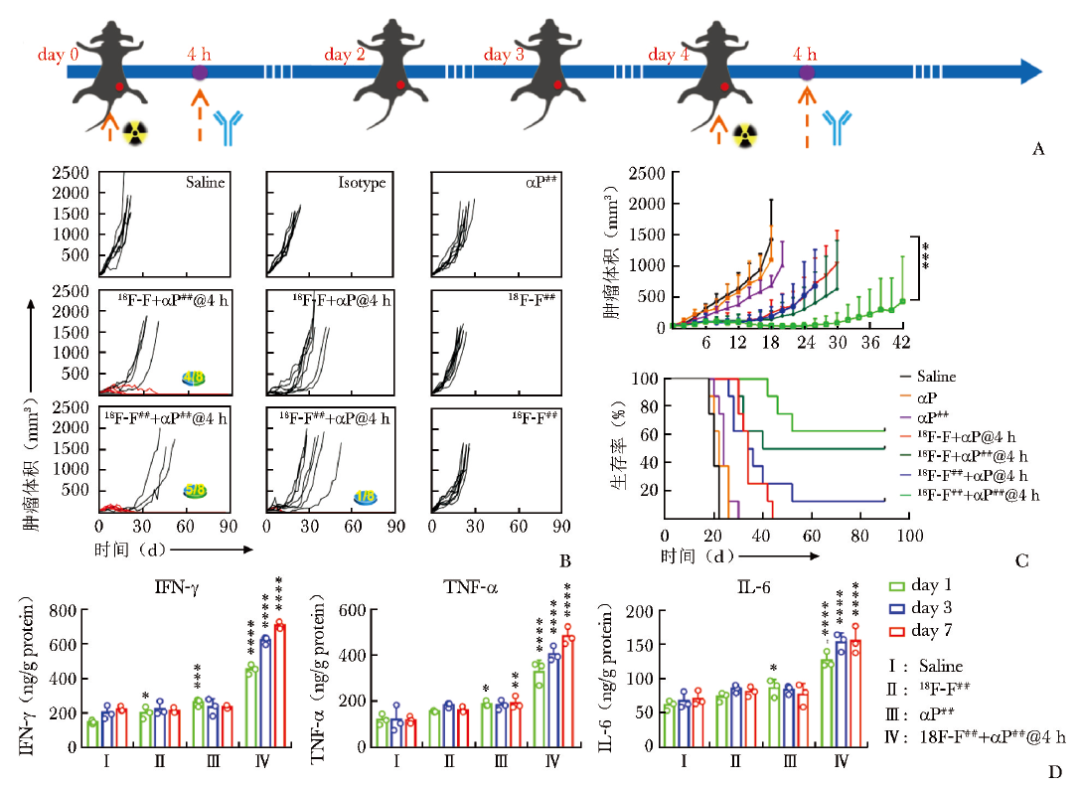
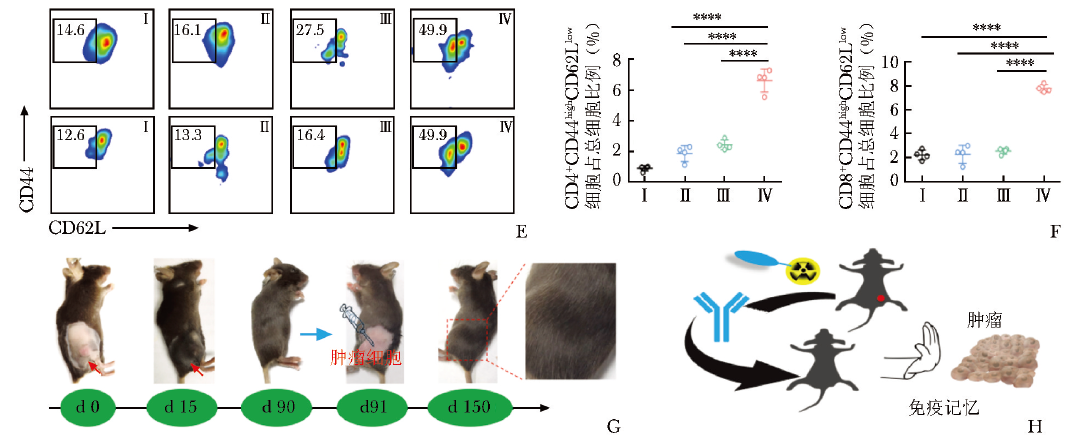
图1 2-[18F]FDG联合PD-L1 ICI治疗可显著延缓肿瘤生长,提高荷瘤小鼠总生存期
A.MC38荷瘤小鼠的治疗程序和时间表示意图;B.不同治疗组MC38荷瘤小鼠的个体肿瘤生长情况以及90 d存活率(αP指PD-L1抗体剂量为10 mg/kg,αP##指PD-L1抗体剂量为20 mg/kg;18F-F指2-[18F]FDG剂量为925 MBq/kg,18F-F##指2-[18F]FDG剂量为1850 MBq/kg;@4 h指PD-L1抗体与2-[18F]FDG的给药时间窗为4 h);C.2-[18F]FDG诱导MC38荷瘤小鼠免疫治疗的时间依赖性肿瘤生长曲线和生存曲线;D.ELISA法检测血液中细胞因子IFN-γ、TNF-α、IL-6水平的动态变化;E.记忆性T细胞浸润的流式细胞术分析(CD4+CD44highCD62Llow和CD8+CD44highCD62Llow);F.用FlowJo v10软件定量分析脾脏总细胞中CD4+CD44highCD62Llow细胞和CD8+CD44highCD62Llow细胞比例;G.治愈小鼠的左后侧在第91天再次接种MC38细胞,并监测至第150天;H.2-[18F]FDG联合PD-L1单抗可增强持久免疫记忆
PD-L1:程序性死亡受体配体1;IFN:干扰素;TNF:肿瘤坏死因子;IL:白细胞介素;*P<0.05;**P<0.01;***P<0.001;****P<0.0001
进一步研究发现,联合治疗组小鼠脾脏内的TEM(CD8+/CD4+CD44highCD62Llow)维持在较高水平,提示小鼠有免疫记忆产生;在治疗过程中,血清中促炎细胞因子IFN-γ、肿瘤坏死因子(TNF) -α及IL-6,TIME中Th1及CTLs于7 d内维持在较高水平,而单独2-[18F]FDG或PD-L1治疗组的细胞因子水平和CTLs水平轻度增高并于7 d内耗竭。Tregs(CD45+CD4+FOXP3+)、M2型巨噬细胞(CD206+F4/80+)及MDSC(CD45+CD11b+Gr-1+)仅在联合治疗组中下降,M1型巨噬细胞(iNOS+F4/80+)和DCs(CD80+CD86+)则显著上调。
以上发现提示联合治疗对TIME的调控作用,相比于单独治疗组,联合治疗不仅更大程度地激活了小鼠的适应性免疫,还降低了免疫抑制性细胞水平,改善了免疫治疗的耐药性。
本课题组进一步探究了2-[18F]FDG的免疫调节作用,发现2-[18F]FDG在体外可导致DNA损伤,但仍保持复制及自我修复能力(图2A,2B)。
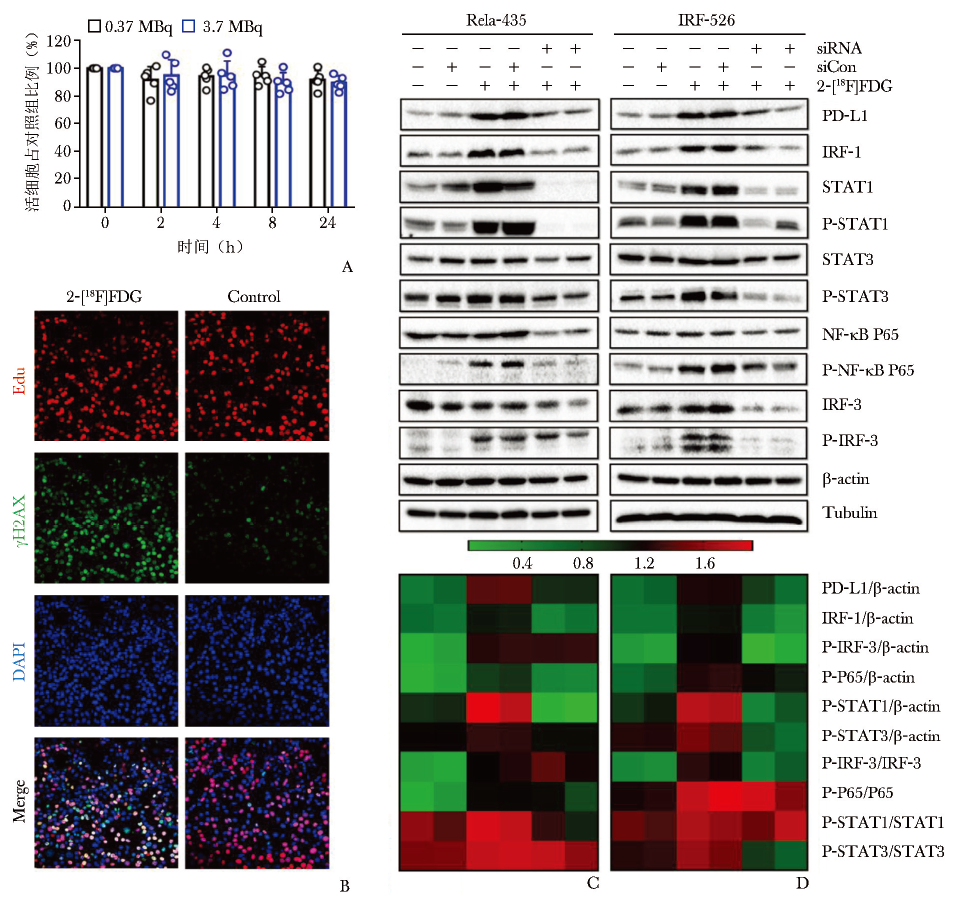
图2 2-[18F]FDG诱导的PD-L1上调通过NF-κB/IRF3途径介导
A.MC38细胞与2-[18F]FDG共孵育不同时间后的细胞活力检测;B.用2-[18F]FDG (3.7 MBq) 照射MC38细胞24 h,用γH2AX和EdU染色检测DNA DSB和DNA修复水平;C、D.2-[18F]FDG激活NF-κB和IRF3信号通路的验证[56]
PD-L1:同图1
同时,2-[18F]FDG通过激活NF-κB P65通路,与IRF3相互作用,促进PD-L1的转录,在体外呈时间及剂量依赖性上调;进一步研究发现,STAT1、STAT3和IRF1的敲低影响NF-κB P65的磷酸化(图2C, 2D及图3),因此PD-L1的上调也受STAT1/3-IRF1通路调控[56]。
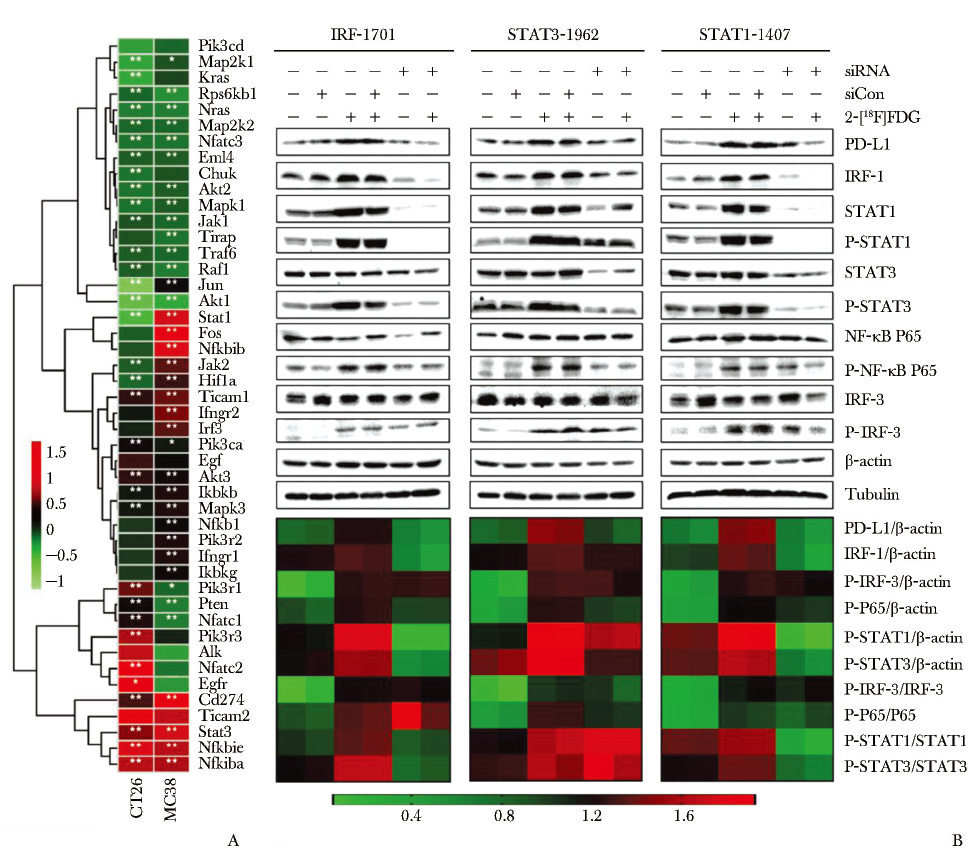
图3 2-[18F]FDG诱导的PD-L1上调与经典STAT1/3-IRF1通路相关
A.与2-[18F]FDG共孵育24 h后肿瘤细胞中DEGs的热图;B.2-[18F]FDG激活STAT1/3和IRF1信号通路与PD-L1上调相关[56]
PD-L1:同图1
本课题组亦探索了纯发射γ射线的核素99mTc标记的RGD对TIME的调节作用,发现同2-[18F]FDG一样,99mTc刺激PD-L1在多种肿瘤细胞系中上调。随后比较了不同联合治疗方案的疗效,发现18.5 MBq或37 MBq 99mTc-RGD联合400 μg PD-L1抗体在4 h时间窗给药效果最佳,部分小鼠的肿瘤完全治愈(6/8)且90 d内无复发[57]。
3 小结与展望
总结近5年发表的放射性药物联合ICI协同抗肿瘤治疗的临床前研究发现,该类研究主要围绕治疗核素展开,包括经典的β核素(177Lu、90Y)以及α核素(211At、225Ac)等(表1)。
表1 近5年发表的放射性药物联合ICI治疗的临床前研究


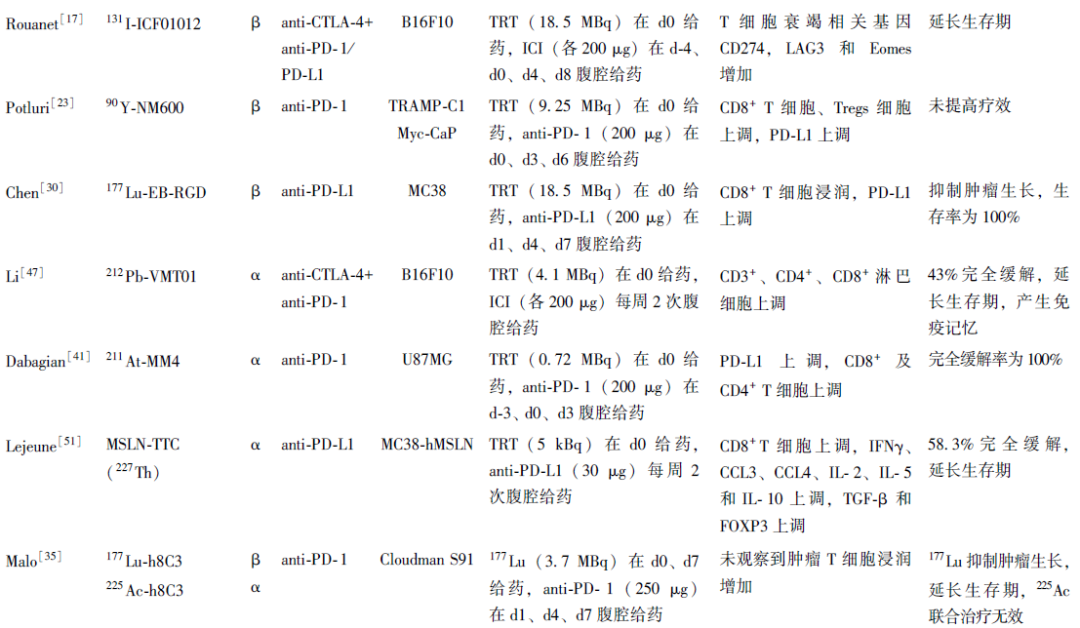

TRT上调了PD-L1表达,增加CTLs及Th1细胞浸润,为后续ICI治疗提供了免疫原性微环境。联合治疗可进一步调控TIME,增加促炎细胞因子、TEM、M1巨噬细胞等浸润,下调Tregs及MDSC,有效抑制肿瘤生长,延长生存期,产生免疫记忆效应,部分研究甚至达到完全缓解。然而,也有研究指出,联合治疗未能正向调控TIME,未能提高TRT的疗效[23,35,48]。
本团队既往研究发现,131I刺激了肿瘤细胞PD-L1表达呈时间及剂量依赖性上调[58]。故在此基础上,进一步选用β核素177Lu及64Cu进行探索,发现64Cu不仅可用于诊断,还具有治疗潜力[59],用64Cu-DER联合PD-L1抗体的协同抗肿瘤治疗大大提升了64Cu-TRT的疗效[32]。
此外,基于2-[18F]FDG的相关研究结果[53-55],本研究团队发现,2-[18F]FDG诱导PD-L1的表达上调由NF-κB/IRF3和STAT1/3-IRF1信号通路介导,与DNA损伤通路激活有关,且放射性刺激与PD-L1抗体的给药时间窗对于治疗效果至关重要。在2-[18F]FDG刺激下,PD-L1上调需要一定时间,而18F半衰期短,难以长时间诱导PD-L1上调,因此4 h的时间窗最为合适[56]。
综上所述,诊断放射性药物联合PD-L1抗体的免疫治疗新范式可以调控TIME,显著提高疗效。与TRT相比,诊断放射性药物具有多种优势,包括可及性高,制备简单及血液毒性小等,如能将诊断性核素标记的PET/SPECT示踪剂应用于肿瘤联合治疗,将极大拓宽其应用范围,改善现有治疗性核素产量有限,特别是我国目前严重依赖进口的现状,拓展传统的肿瘤治疗模式。
因此,未来研究中应进一步拓展诊断放射性药物联合免疫治疗的组合,探索不同核素对肿瘤细胞及微环境中其他细胞的影响,促进此种治疗新范式的临床转化。
参考文献
[1]FDA approves anti-LAG3 checkpoint[J]. Nat Biotechnol, 2022,40:625.
[2]Yi M, Zheng X, Niu M, et al. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions[J]. Mol Cancer, 2022,21:28.
[3]de Miguel M,Calvo E. Clinical Challenges of Immune Checkpoint Inhibitors[J]. Cancer Cell, 2020,38:326-333.
[4]Fradet Y, Bellmunt J, Vaughn DJ, et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up[J]. Ann Oncol, 2019,30:970-976.
[5]McLaughlin M, Patin EC, Pedersen M, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy[J]. Nat Rev Cancer, 2020,20:203-217.
[6]Pouget JP, Lozza C, Deshayes E, et al. Introduction to radiobiology of targeted radionuclide therapy[J]. Front Med (Lausanne), 2015,2:12.
[7]Deng L, Liang H, Xu M, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors[J]. Immunity, 2014,41:843-852.
[8]Zhang X, Zhang H, Zhang J, et al. The paradoxical role of radiation-induced cGAS-STING signalling network in tumour immunity[J]. Immunology, 2023,168:375-388.
[9]Lan Y, Moustafa M, Knoll M, et al. Simultaneous targeting of TGF-beta/PD-L1 synergizes with radiotherapy by reprogramming the tumor microenvironment to overcome immune evasion[J]. Cancer Cell, 2021,39:1388-403. e10.
[10]Sha CM, Lehrer EJ, Hwang C, et al. Toxicity in combination immune checkpoint inhibitor and radiation therapy: A systematic review and meta-analysis[J]. Radiother Oncol, 2020,151:141-148.
[11]Procureur A, Simonaggio A, Bibault JE, et al. Enhance the Immune Checkpoint Inhibitors Efficacy with Radiotherapy Induced Immunogenic Cell Death: A Comprehensive Review and Latest Developments[J]. Cancers (Basel), 2021,13:678.
[12]Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer[J]. Nature, 2015,520:373-377.
[13]Formenti SC,Demaria S. Systemic effects of local radiotherapy[J]. Lancet Oncol, 2009,10:718-726.
[14]Formenti SC, Rudqvist NP, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade[J]. Nat Med, 2018,24:1845-1851.
[15]Kleinendorst SC, Oosterwijk E, Bussink J, et al. Combining Targeted Radionuclide Therapy and Immune Checkpoint Inhibition for Cancer Treatment[J]. Clin Cancer Res, 2022,28:3652-3657.
[16]Sun Q, Li J, Ding Z, et al. Radiopharmaceuticals heat anti-tumor immunity[J]. Theranostics, 2023,13:767-786.
[17]Rouanet J, Benboubker V, Akil H, et al. Immune checkpoint inhibitors reverse tolerogenic mechanisms induced by melanoma targeted radionuclide therapy[J]. Cancer Immunol Immunother, 2020,69:2075-2088.
[18]Jagodinsky JC, Jin WJ, Bates AM, et al. Temporal analysis of type 1 interferon activation in tumor cells following external beam radiotherapy or targeted radionuclide therapy[J]. Theranostics, 2021,11:6120-6137.
[19]Patel RB, Hernandez R, Carlson P, et al. Low-dose targeted radionuclide therapy renders immunologically cold tumors responsive to immune checkpoint blockade[J]. Sci Transl Med, 2021,13:eabb3631.
[20]Grzmil M, Boersema P, Sharma A, et al. Comparative analysis of cancer cell responses to targeted radionuclide therapy (TRT) and external beam radiotherapy (EBRT)[J]. J Hematol Oncol, 2022,15:123.
[21]Zhang J, Yang M, Fan X, et al. Biomimetic radiosensitizers unlock radiogenetics for local interstitial radiotherapy to activate systematic immune responses and resist tumor metastasis[J]. J Nanobiotechnology, 2022,20:103.
[22]Brown R, Hernandez R, Grudzinski JJ, et al. Ability of Molecular Targeted Radionucleotide Therapy and Anti-CTLA-4 to Prevent Spontaneous Metastases in a Preclinical Lewis Lung Carcinoma Model[J]. Int J Radiat Oncol Biol Phys, 2019,105:E498-E499.
[23]Potluri HK, Ferreira CA, Grudzinski J, et al. Antitumor efficacy of 90Y-NM600 targeted radionuclide therapy and PD-1 blockade is limited by regulatory T cells in murine prostate tumors[J]. J Immunother Cancer, 2022,10:e005060.
[24]Lutetium (Lu-177) Dotatate Approved by FDA[J]. Cancer Discov, 2018,8:OF2.
[25]Fallah J, Agrawal S, Gittleman H, et al. FDA Approval Summary: lutetium (Lu-177) vipivotide tetraxetan for patients with metastatic castration-resistant prostate cancer[J]. Clin Cancer Res, 2023,29:1651-1657.
[26]Kim C, Liu SV, Subramaniam DS, et al. Phase Ⅰ study of the 177Lu-DOTA(0)-Tyr(3)-Octreotate (lutathera) in combination with nivolumab in patients with neuroendocrine tumors of the lung[J]. J Immunother Cancer, 2020,8:e000980.
[27]Ferdinandus J, Fendler WP, Lueckerath K, et al. Response to Combined Peptide Receptor Radionuclide Therapy and Checkpoint Immunotherapy with Ipilimumab Plus Nivolumab in Metastatic Merkel Cell Carcinoma[J]. J Nucl Med, 2022,63:396-398.
[28]Lin AL, Tabar V, Young RJ, et al. Synergism of Checkpoint Inhibitors and Peptide Receptor Radionuclide Therapy in the Treatment of Pituitary Carcinoma[J]. J Endocr Soc, 2021,5:bvab133.
[29]Prasad V, Zengerling F, Steinacker JP, et al. First Experiences with 177Lu-PSMA Therapy in Combination with Pembrolizumab or After Pretreatment with Olaparib in Single Patients[J]. J Nucl Med, 2021,62:975-978.
[30]Chen H, Zhao L, Fu K, et al. Integrin αυβ3-targeted radionuclide therapy combined with immune checkpoint blockade immunotherapy synergis-tically enhances anti-tumor efficacy[J]. Theranostics, 2019,9:7948-7960.
[31]Wen XJ, Zeng XY, Shi CR, et al. Optimum combination of radiopharmaceuticals-based targeting-triggering-therapy effect and PD-L1 blockade immunotherapy[J]. Adv Ther, 2022,6:2200193.
[32]Wen X, Zeng X, Liu J, et al. Synergism of 64Cu-Labeled RGD with Anti-PD-L1 Immunotherapy for the Long-Acting Antitumor Effect[J]. Bioconjug Chem, 2022,33:2170-2179
[33]Guzik P, Siwowska K, Fang HY, et al. Promising potential of [177Lu]Lu-DOTA-folate to enhance tumor response to immunotherapy-a preclinical study using a syngeneic breast cancer model[J]. Eur J Nucl Med Mol Imaging, 2021,48:984-994.
[34]Ren J, Xu M, Chen J, et al. PET imaging facilitates antibody screening for synergistic radioimmunotherapy with a 177Lu-labeled alphaPD-L1 antibody[J]. Theranostics, 2021,11:304-315.
[35]Malo ME, Allen KJH, Jiao R, et al. Mechanistic Insights into Synergy between Melanin-Targeting Radioimmun-otherapy and Immunotherapy in Experimental Melanoma[J]. Int J Mol Sci, 2020,21:8721.
[36]Choi J, Beaino W, Fecek RJ, et al. Combined VLA-4-Targeted Radionuclide Therapy and Immunotherapy in a Mouse Model of Melanoma[J]. J Nucl Med, 2018,59:1843-1849.
[37]Vito A, Rathmann S, Mercanti N, et al. Combined Radionuclide Therapy and Immunotherapy for Treatment of Triple Negative Breast Cancer[J]. Int J Mol Sci, 2021,22:4843.
[38]Stap J, Krawczyk PM, Van Oven CH, et al. Induction of linear tracks of DNA double-strand breaks by alpha-particle irradiation of cells[J]. Nat Methods, 2008,5:261-266.
[39]Czernin J, Current K, Mona CE, et al. Immune-Checkpoint Blockade Enhances 225Ac-PSMA617 Efficacy in a Mouse Model of Prostate Cancer[J]. J Nucl Med, 2021,62:228-231.
[40]Josefsson A, Nedrow JR, Park S, et al. Combining alpha-particle radiopharmaceutical therapy using Actinium-225 and immunotherapy with anti-PD-L1 antibodies in a murine immunocompetent metastatic breast cancer model[J]. Cancer Res, 2016,76:3052.
[41]Dabagian H, Taghvaee T, Martorano P, et al. PARP Targeted Alpha-Particle Therapy Enhances Response to PD-1 Immune-Checkpoint Blockade in a Syngeneic Mouse Model of Glioblastoma[J]. ACS Pharmacol Transl Sci, 2021,4:344-351.
[42]Zhang J, Li F, Yin Y, et al. Alpha radionuclide-chelated radioimmunotherapy promoters enable local radiotherapy/chemodynamic therapy to discourage cancer progression[J]. Biomater Res, 2022,26:44.
[43]Malamas AS, Gameiro SR, Knudson KM, et al. Sublethal exposure to alpha radiation 223Ra dichloride) enhances various carcinomas' sensitivity to lysis by antigen-specific cytotoxic T lymphocytes through calreticulin-mediated immunogenic modulation[J]. Oncotarget, 2016,7:86937-86947.
[44]Creemers JHA, van der Doelen MJ, van Wilpe S, et al. Immunophenotyping Reveals Longitudinal Changes in Circulating Immune Cells During Radium-223 Therapy in Patients With Metastatic Castration-Resistant Prostate Cancer[J]. Front Oncol, 2021,11:667658.
[45]Vardaki I, Corn P, Gentile E, et al. Radium-223 Treatment Increases Immune Checkpoint Expression in Extracellular Vesicles from the Metastatic Prostate Cancer Bone Microenvironment[J]. Clin Cancer Res, 2021,27:3253-3264.
[46]Fong L, Morris MJ, Sartor O, et al. A Phase Ib Study of Atezolizumab with Radium-223 Dichloride in Men with Metastatic Castration-Resistant Prostate Cancer[J]. Clin Cancer Res, 2021,27:4746-4756.
[47]Li M, Liu D, Lee D, et al. Targeted Alpha-Particle Radiotherapy and Immune Checkpoint Inhibitors Induces Cooperative Inhibition on Tumor Growth of Malignant Melanoma[J]. Cancers (Basel), 2021,13:3676.
[48]Nosanchuk JD, Jeyakumar A, Ray A, et al. Structure-function analysis and therapeutic efficacy of antibodies to fungal melanin for melanoma radioimmunotherapy[J]. Sci Rep, 2018,8:5466.
[49]Perrin J, Capitao M, Allard M, et al. Targeted Alpha Particle Therapy Remodels the Tumor Microenvironment and Improves Efficacy of Immunotherapy[J]. Int J Radiat Oncol Biol Phys, 2022,112:790-801.
[50]Gorin JB, Menager J, Gouard S, et al. Antitumor immunity induced after alpha irradiation[J]. Neoplasia, 2014,16:319-328.
[51]Lejeune P, Cruciani V, Berg-Larsen A, et al. Immunostimulatory effects of targeted thorium-227 conjugates as single agent and in combination with anti-PD-L1 therapy[J]. J Immunother Cancer, 2021,9:e002387.
[52]Hagemann UB, Ellingsen C, Schuhmacher J, et al. Mesothelin-Targeted Thorium-227 Conjugate (MSLN-TTC): Preclinical Evaluation of a New Targeted Alpha Therapy for Mesothelin-Positive Cancers[J]. Clin Cancer Res, 2019,25:4723-4734.
[53]Moadel RM, Nguyen AV, Lin EY, et al. Positron emission tomography agent 2-deoxy-2-[18F]fluoro-D-glucose has a therapeutic potential in breast cancer[J]. Breast Cancer Res, 2003,5:R199-R205.
[54]Moadel RM, Weldon RH, Katz EB, et al. Positherapy: targeted nuclear therapy of breast cancer with 18F-2-deoxy-2-fluoro-D-glucose[J]. Cancer Res, 2005,65:698-702.
[55]Fang S, Wang J, Jiang H, et al. Experimental study on the therapeutic effect of positron emission tomography agent[18F]-labeled 2-deoxy-2-fluoro-d-glucose in a colon cancer mouse model[J]. Cancer Biother Radiopharm, 2010,25:733-740.
[56]Wen X, Shi C, Zeng X, et al. A Paradigm of Cancer Immunotherapy Based on 2-[18F]FDG and Anti-PD-L1 mAb Combination to Enhance the Antitumor Effect[J]. Clin Cancer Res, 2022,28:2923-2937.
[57]文雪君, 周吴昊, 郭志德, 等. 整合素αυβ3 靶向放射性药物99mTc-RGD联合抗PD-L1肿瘤免疫治疗增强抗肿瘤效果的研究[J]. 协和医学杂志, 2023, 14:766-773.
[58]Wen X, Zeng X, Cheng X, et al. PD-L1-Targeted Radionuclide Therapy Combined with alpha PD-L1 Antibody Immunotherapy Synergistically Improves the Antitumor Effect[J]. Mol Pharm, 2022,19:3612-3622.
[59]Gutfilen B, Souza SA,Valentini G. Copper-64: a real theranostic agent[J]. Drug Des Devel Ther, 2018,12:3235-3245.


